-
Industrial Chemistry

Aluminum vs Alloy
Are you facing the dilemma of choosing between alloy and aluminum for your next project? This decision can significantly impact the outcome, whether you're constructing a towering skyscraper, designing an efficient aerospace component, or even selecting the perfect cookware for your kitchen. To make an informed choice, let's delve into the differences between these two materials. Alloy, in essence, is an amalgamation of two or more elements, often including metals and sometimes non-metals, meticulously engineered to exhibit specific properties. These properties can range from remarkable strength and durability to superior corrosion resistance and electrical conductivity. Alloys span a broad spectrum, encompassing stalwarts like steel, brass, bronze, and high-strength alloys. Each alloy type boasts unique characteristics, making them suitable for diverse industries and applications. Aluminum, on the other hand, is a lightweight metal, denoted by the symbol Al, naturally occurring in the Earth's crust. It stands out for its low density, rendering it about one-third as heavy as steel. This characteristic makes aluminum a favored choice in industries where weight reduction is paramount, such as aerospace and automotive. Yet, aluminum's appeal extends beyond its lightweight nature; it boasts excellent thermal and electrical conductivity and corrosion resistance, further enhancing its versatility.
-
Accessories Sports

Firm Ground vs Soft Ground
When it comes to outdoor pursuits, your choice of footwear can significantly impact your performance, comfort, and safety. Soft ground and firm ground terrains pose unique challenges, and selecting the right footwear is crucial for a seamless experience. Soft Ground: This category encompasses terrains such as muddy trails, wet grass, and sandy beaches. These surfaces are characterized by their malleability, where your foot can sink or slide to varying degrees. Soft ground shoes are designed with aggressive tread patterns, ample cushioning, and reinforced ankle support. These features provide excellent traction, impact absorption, and stability, making them ideal for activities like trail running, hiking through muddy paths, and other adventures on slippery or uneven surfaces. Firm Ground: Firm ground terrain consists of stable and unyielding surfaces like well-maintained sports fields, rocky paths, and synthetic tracks. Here, your primary concern is maintaining grip without the risk of sinking. Firm ground footwear typically features moderate tread patterns, responsive designs, and a balance between support and natural motion. These characteristics cater to activities such as running on tracks, casual walks on solid paths, and sports like tennis or volleyball.
-
Organic Chemistry

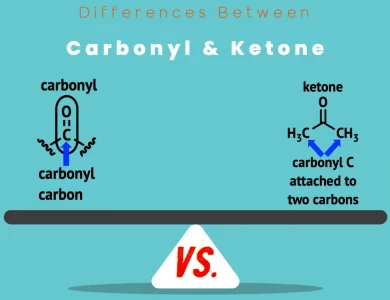
Ketone vs Carbonyl
When it comes to organic chemistry, understanding the nuances between carbonyl compounds and ketones is pivotal. Carbonyl compounds, characterized by a carbon atom double-bonded to an oxygen atom (C=O), encompass a diverse range of molecules, including aldehydes, ketones, carboxylic acids, esters, and amides. In contrast, ketones represent a specific subset within this category, featuring the C(=O) bond situated between two carbon-containing groups. This structural divergence sets the stage for differences in their physical properties, such as boiling and melting points, as well as their reactivity patterns. While both carbonyl compounds and ketones partake in nucleophilic addition reactions, ketones generally exhibit greater resistance to oxidation. This resistance renders ketones invaluable in various chemical processes and industrial applications. To gain a comprehensive understanding of these distinctions, read on to explore the world of carbonyl vs ketone compounds.
-
Polymer Chemistry

Silicone vs Rubber
Curious about the distinctions between rubber and silicone? These versatile materials play essential roles in various industries. Rubber, derived from natural or synthetic sources, is known for its flexibility and impact resistance. On the other hand, silicone, a synthetic polymer with a silicon-oxygen backbone, excels in extreme heat resistance and versatility across temperature ranges. The heat resistance of silicone makes it suitable for applications involving high temperatures, while rubber offers resilience and flexibility. While rubber is prized for its familiarity and affordability, silicone's exceptional properties come at a higher cost. When choosing between them, consider factors such as your application's needs, budget, and environmental considerations. Whether you're looking for heat resistance, flexibility, or chemical compatibility, understanding these differences will empower you to make an informed decision that aligns with your project's requirements. Explore the world of rubber and silicone, and make the right choice for your unique needs.
-
Organic Chemistry

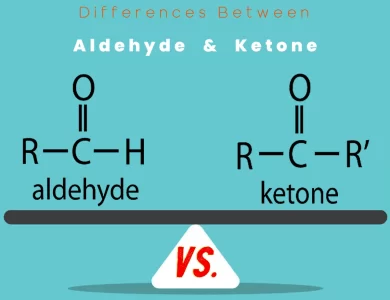
Ketone vs Aldehyde
Delve into the captivating world of organic chemistry as we unravel the intricate disparities between aldehyde and ketone molecules. These two distinct classes of compounds, both featuring the carbonyl functional group, possess unique structural arrangements and reactivity patterns that set them apart. Aldehydes showcase a carbonyl group bonded to a hydrogen atom, while ketones boast a carbonyl group positioned between two carbon atoms. This seemingly subtle difference leads to profound variations in their chemical behavior and applications. In the realm of reactivity, aldehydes are known for their susceptibility to oxidation, readily transforming into carboxylic acids. On the other hand, ketones exhibit a remarkable resistance to oxidation due to the absence of an active hydrogen atom. The role of these compounds extends beyond reactions, touching areas such as fragrances, flavor compounds, solvents, and even pharmaceutical synthesis. Aldehydes find their place in perfumery and preservation, while ketones excel in applications involving solubility, drug development, and materials science. Our comprehensive exploration navigates through the intricacies of aldehydes and ketones, shedding light on their distinct roles in chemistry and industries. Whether you're a chemistry enthusiast, student, or industry professional, understanding these differences offers a deeper appreciation for the intricate dance of molecules that shape our world. Join us as we embark on a journey of discovery, uncovering the fascinating disparities and applications of aldehyde and ketone molecules in our comprehensive blog, "Differences Between Aldehyde vs Ketone."
-
Organic Chemistry

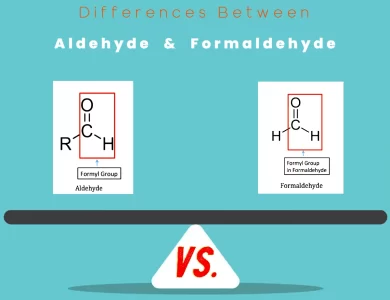
Formaldehyde vs Aldehyde
AspectAldehydeFormaldehydeChemical StructureContains a carbonyl group (C=O) bonded to a carbon and a hydrogen atom.Contains a carbonyl group (H-C=O) bonded to hydrogen and oxygen atoms.Physical StateCan exist as both liquids and gases at room temperature.Exists as a colorless, pungent-smelling gas at room temperature.OdorVaries in odor, from fruity to sharp, depending on the specific compound.Has a distinct pungent and strong odor.Boiling PointBoiling points generally higher than those of corresponding alcohols.Boiling point is relatively low due to its small molecular size.ReactivityParticipates in nucleophilic addition and oxidation reactions.Reacts in nucleophilic addition reactions and can crosslink with proteins.ApplicationsUsed in perfumery, flavoring, pharmaceuticals, and organic synthesis.Employed as a disinfectant, preservative, and in the production of materials.Biochemical RolePlays a role in metabolism and signaling pathways within living organisms.Not a significant participant in biological processes.Polymer ChemistryLimited role in polymer chemistry compared to other functional groups.Essential for the production of formaldehyde-based resins used in construction and materials manufacturing.Health ImpactGenerally safe in small amounts but can cause irritation or allergies in some individuals.Prolonged exposure can lead to respiratory irritation, sensitization, and health issues.Environmental ImpactCan contribute to air pollution when emitted as pollutants.Formaldehyde emissions can contribute to air pollution and ground-level ozone formation.Analytical TechniquesDetected and quantified using gas chromatography, HPLC, and …
-
Sports

Freerunning vs Parkour
Are you ready to dive into the exhilarating world of movement and athleticism? Parkour and Freerunning are two captivating disciplines that have taken the world by storm. In this exploration, we'll unveil the key differences that set Parkour and Freerunning apart, allowing you to gain a deeper understanding of each practice and make an informed choice based on your interests and aspirations. At the core, Parkour embodies the essence of efficiency. Originating in early 20th-century France, Parkour, developed by David Belle and his friends, focuses on traversing obstacles swiftly and efficiently using one's body. The philosophy revolves around getting from point A to point B in the most direct and fluid manner possible. It emphasizes practicality, mastering fundamental movements, and honing your physical capabilities for real-world applications. In contrast, Freerunning, which emerged as an extension of Parkour in the late 20th century, introduces a creative and expressive dimension to the discipline. Sebastien Foucan, another influential figure in the movement, brought forth the concept of Freerunning, which seamlessly blends acrobatic movements and flips into the practice. Freerunning encourages athletes to explore their physical limits, experiment with various tricks, and infuse their personal flair into their routines. It's an artistic and dynamic expression of movement that transforms the environment into a personal playground.
-
Organic Chemistry

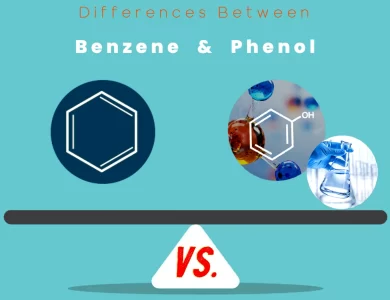
Phenol vs Benzene
Delving into the realm of organic chemistry, the disparities between benzene and phenol come to life as two aromatic compounds take center stage. Benzene, known for its resonance-driven stability, boasts a hexagonal ring structure intertwined with alternating single and double bonds. In contrast, phenol adds a twist to the narrative with a hydroxyl group adorning its aromatic ring, enhancing reactivity and versatility. While benzene leans towards electrophilic aromatic substitutions, phenol's hydroxyl group invites both substitution and addition reactions into its chemistry. Beyond reactivity, these compounds unveil divergent physical properties. Benzene exhibits a lower boiling point influenced by London dispersion forces, while phenol's hydrogen bonding elevates its boiling point. The applications of benzene span industries like petrochemicals and pharmaceuticals, while phenol finds its niche in antiseptics, plastics, and resins. Despite their aromatic commonality, their impacts diverge—benzene, a carcinogen with environmental concerns, and phenol, a safer alternative with less toxicity. The differences between benzene and phenol are more than skin deep; they reflect the intricacies of molecular arrangement and chemical behavior. This exploration unravels their unique stories, highlighting their roles in the intricate tapestry of chemistry and inspiring curiosity about the diverse world of compounds.
-
Martial Arts

Difference Between MMA and UFC
In the realm of combat sports, the terms "UFC" and "MMA" often intertwine, but they represent different facets of the thrilling world of mixed martial arts. Let's dive into the nuanced differences that set UFC and MMA apart. MMA (Mixed Martial Arts): MMA, an abbreviation for Mixed Martial Arts, serves as the comprehensive sport that encompasses a vast array of fighting styles and techniques. It is a combat sport where fighters utilize a combination of striking (such as boxing, kickboxing, and Muay Thai) and grappling (like Brazilian Jiu-Jitsu and wrestling) to compete in a regulated environment. MMA represents the broader landscape of combat sports, showcasing various promotions, fighters, weight classes, and rulesets worldwide. UFC (Ultimate Fighting Championship): On the other hand, the UFC, or Ultimate Fighting Championship, is a specific and globally renowned promotion within the realm of MMA. Established in 1993, the UFC has played a pivotal role in popularizing MMA. It hosts high-profile events featuring elite fighters from around the world, crowning champions in multiple weight classes. While the UFC is a dominant force in MMA, it's essential to recognize that MMA extends beyond the confines of this particular promotion. Other organizations, such as Bellator, ONE Championship, and PFL, contribute to the rich tapestry of mixed martial arts. Understanding these distinctions is crucial for anyone delving into the captivating world of combat sports, whether as a fan, aspiring fighter, or someone simply curious about the dynamic sport of MMA.
-
Inorganic Chemistry

Graphite vs Carbon
Explore the intricate variations between carbon and graphite in this insightful exploration. Carbon, the versatile sixth element, takes on multifaceted forms, from the brilliance of diamonds to the opacity of amorphous substances. On the other hand, graphite showcases a layered elegance, composed of interconnected hexagonal rings that grant it exceptional conductivity and lubricity. These elemental siblings offer distinct properties, bonding types, and even optical characteristics. While carbon displays diverse bonding, graphite's covalent bonds within layers and weak interlayer forces define its conductivity and appearance. In terms of applications, carbon finds its place in a wide range of industries, while graphite excels in roles such as lubricants, electrodes, and pencils. Whether you're intrigued by their mechanical strengths or their thermal conductivities, delving into their differences illuminates the fascinating world of elements and materials science. Join us on a journey to unravel the mysteries that set carbon and graphite apart, shedding light on their diverse roles in shaping our technological landscape.