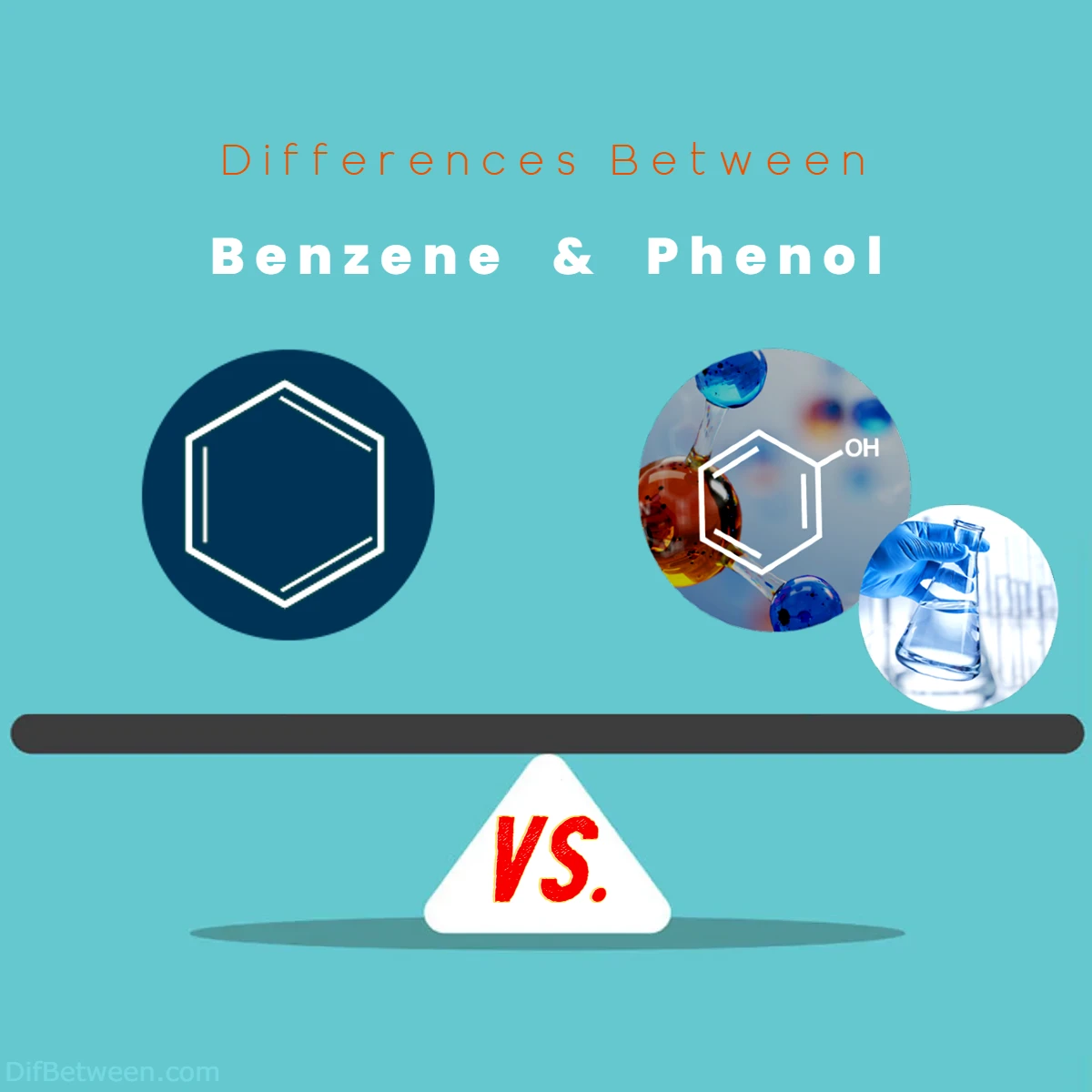
| Aspect | Benzene | Phenol |
|---|---|---|
| Chemical Formula | C6H6 | C6H5OH |
| Structural Feature | Hexagonal ring with alternating single/double bonds | Hexagonal ring with a hydroxyl (-OH) group |
| Aromaticity | Exhibits resonance-driven aromatic stability | Aromatic with added reactivity due to -OH group |
| Reactivity | Prefers electrophilic aromatic substitution | Participates in both substitution and addition reactions |
| Physical Properties | Lower boiling point due to London dispersion forces | Higher boiling point due to hydrogen bonding |
| Applications | Precursor for polymers and chemicals | Antiseptics, disinfectants, plastics, and resins |
| Toxicity | Classified as a carcinogen, poses health risks | Less hazardous but can cause irritation |
| Environmental Impact | Contributes to air pollution and VOC emissions | Lower environmental impact compared to benzene |
| Functionalization | Substitution reactions lead to diverse derivatives | Unique reactions due to hydroxyl (-OH) group |
Picture a realm where atoms dance in intricate arrangements, giving rise to molecules that hold secrets to diverse reactivity, applications, and impacts. Our adventure today centers around two intriguing protagonists: the aromatic molecules known as benzene and phenol.Benzene, with its enchanting hexagonal ring structure, stands as a symbol of resonance-driven stability, casting a spell of aromatic allure. Meanwhile, phenol enters the scene with a twist—a hydroxyl group that infuses reactivity into its aromatic framework.
Differences Between Benzene and Phenol
Benzene and phenol, two aromatic compounds, exhibit significant differences in their structures, reactivity, and applications. Benzene boasts a hexagonal ring with alternating single and double bonds, showcasing resonance-driven stability. In contrast, phenol’s structure features a hydroxyl group attached to the aromatic ring, enhancing its reactivity and applications. While benzene prefers electrophilic aromatic substitution reactions, phenol participates in both substitution and addition reactions due to its hydroxyl group. These disparities extend to their physical properties, toxicity profiles, and environmental impacts. Benzene is known to be carcinogenic and environmentally harmful, while phenol offers relatively lower toxicity and serves as a safer alternative. Exploring these contrasts unveils the distinct roles these compounds play in the realms of chemistry and industry.
1. Structural Foundation: A Tale of Rings and Groups
Benzene: Aromatic Delight
At the heart of benzene’s allure lies its hexagonal ring structure, consisting of six carbon atoms bonded harmoniously in a ring, with alternating single and double bonds. This arrangement, known as the “resonance hybrid” model, paints benzene as a molecule that oscillates between various resonance structures, bestowing upon it unparalleled stability. The electrons in the double bonds are distributed evenly across the ring, creating a cloud of electron density that contributes to its aromaticity.
Benzene’s chemical formula, C6H6, belies its intricate structure, which captivated chemists for years before the advent of modern structural theories. The symmetrical beauty of benzene’s arrangement of carbon and hydrogen atoms endows it with a mesmerizing flat, planar geometry.
Phenol: Hydroxyl-Flavored Twist
Phenol, on the other hand, carries a unique twist in its structure that sets it apart from benzene. It’s not just a simple aromatic ring; phenol boasts an attached hydroxyl group (OH) at one of its carbon positions. This functional group introduces polarity and significantly influences the compound’s behavior and reactivity.
The chemical formula of phenol, C6H5OH, mirrors its distinct structure. The benzene ring stands as the foundation, but the hydroxyl group juts out, conferring phenol with both the characteristics of an aromatic compound and those of an alcohol.
2. Reactivity and Chemical Behavior: Divergence Under the Surface
Benzene: The Stable Centerpiece
Benzene’s structural resonance dance imparts it with extraordinary stability, making it less prone to undergo typical reactions expected of unsaturated hydrocarbons. The delocalized electrons shared among the carbon atoms in the ring form a sort of electron cloud, rendering the double bonds “partial” in nature. This unique electronic configuration makes benzene reluctant to partake in addition reactions, unlike its alkene counterparts. Instead, benzene prefers substitution reactions, where hydrogen atoms are replaced by other groups.
Benzene’s resistance to addition reactions and its inclination towards electrophilic aromatic substitution make it a crucial building block in various industries, including the production of polymers, pharmaceuticals, and chemicals. Its stability and inertness contribute to its longevity, proving that sometimes, it’s the stability within that drives exceptional utility.
Phenol: Hydroxyl Sparks Reactivity
Phenol, crowned with a hydroxyl group, exhibits quite different reactivity compared to its cousin, benzene. The hydroxyl group significantly enhances phenol’s polarity, making it capable of participating in both substitution and addition reactions. The hydroxyl group’s electron-donating nature facilitates electrophilic aromatic substitution, but it also paves the way for reactions typical of alcohols.
Oxidation, for instance, is a noteworthy reaction that phenol undertakes due to the presence of the hydroxyl group. Phenol can be oxidized to yield quinones, which are important intermediates in various biochemical processes. The hydroxyl group’s influence extends even further, granting phenol water solubility and lending itself to applications in antiseptics and disinfectants.
3. Applications: Where Chemistry Meets Utility
Benzene: Behind the Scenes
Benzene might appear modest due to its stability-driven inertness, but it’s a star player behind the scenes. Its resilience to harsh conditions and resistance to rapid reactivity make it a prime candidate for applications where stability is paramount. The petrochemical industry leans on benzene for the production of various polymers, including the ubiquitous polystyrene and nylon. Additionally, benzene acts as a precursor for numerous chemicals, such as aniline, which serves as a building block in dye synthesis.
Benzene’s ring structure isn’t just about stability—it’s about versatility. The world of organic chemistry owes a great deal to benzene for being the cornerstone of aromatic compounds, paving the way for innovations across industries.
Phenol: Medicinal Marvel and Beyond
Phenol’s distinctiveness springs from its hydroxyl group, an attribute that bestows upon it a wide array of applications. One of phenol’s noteworthy roles is its contribution to medicine as an antiseptic and disinfectant. Its germicidal properties make it an invaluable tool for maintaining hygiene and preventing infections.
Beyond medicine, phenol finds utility in the realm of plastics and resins. The production of phenolic resins, celebrated for their heat resistance and durability, is a testament to phenol’s versatility. These resins find their way into consumer goods, automotive parts, and even circuit boards.
4. Physical Properties: The Dance of Intermolecular Forces
Benzene: Aromatic Elegance
The symmetrical structure of benzene and its uniform distribution of electrons give rise to intriguing physical properties. One of the most notable features is its characteristic aroma, which was the inspiration behind its name. Benzene’s alluring scent captures our olfactory senses, making it recognizable even to those unacquainted with the intricacies of organic chemistry.
Benzene’s boiling point, an indicator of intermolecular forces, defies expectations. Considering its molecular weight, one might anticipate a higher boiling point, but benzene’s interactions are dominated by London dispersion forces due to its nonpolar nature. These forces, stemming from temporary fluctuations in electron distribution, allow benzene molecules to attract each other weakly and consequently exhibit a lower boiling point than anticipated.
Phenol: Hydrogen Bonding Complexity
The addition of a hydroxyl group to phenol imparts a remarkable change in its physical properties, especially regarding intermolecular forces. The hydroxyl group introduces hydrogen bonding possibilities between phenol molecules. Hydrogen bonds, being stronger than London dispersion forces, elevate phenol’s boiling point significantly compared to that of benzene.
Phenol’s boiling point rise due to hydrogen bonding isn’t solely for show—it’s a testament to the intriguing dance of intermolecular forces that shapes the behavior of compounds in their various states.
5. Toxicity and Environmental Impact: Weighing the Consequences
Benzene: The Notorious Hydrocarbon
While benzene’s stability and utility are commendable, it’s also important to acknowledge its dark side. Benzene is classified as a carcinogen, and prolonged exposure to high levels of benzene vapor is associated with serious health risks, including the development of leukemia and other blood-related disorders. This toxicity has led to strict regulations in various industries to limit exposure to benzene, especially among workers who handle it regularly.
Benzene’s environmental impact isn’t any less concerning. It’s a volatile organic compound (VOC) that contributes to air pollution and the formation of ground-level ozone, a key component of smog. The recognition of these risks has prompted industries to seek safer alternatives and implement measures to reduce benzene emissions.
Phenol: A Safer Alternative
Phenol’s toxicity profile differs from that of benzene, and it’s generally considered to be less hazardous. While phenol can cause skin and respiratory irritation upon direct contact, its risks are generally lower compared to those posed by benzene. This has led to the use of phenol as a substitute in various applications where the toxic effects of benzene are a concern.
However, it’s worth noting that phenol isn’t entirely without environmental implications. Its persistence in water and potential to bioaccumulate have raised some concerns. Nevertheless, its relatively lower toxicity compared to benzene provides a more favorable outlook in terms of health and safety.
6. Derivatives and Functionalization: Crafting New Possibilities
Benzene: Substitution Reigns
Benzene’s stable and unreactive nature toward addition reactions isn’t a limitation—it’s an opportunity for strategic functionalization. The substitution reactions that benzene prefers have led to the creation of a wide array of benzene derivatives. By replacing one or more hydrogen atoms with various functional groups, chemists can tailor the properties of benzene to suit specific applications.
For instance, the introduction of nitro (-NO2) groups results in nitrobenzene, a compound used in the production of dyes and explosives. Similarly, the attachment of an amino (-NH2) group gives rise to aniline, a vital component in the synthesis of dyes, pharmaceuticals, and rubber chemicals. Benzene’s versatility shines through its derivatives, showcasing the power of controlled functionalization.
Phenol: Altered Dynamics
Phenol’s hydroxyl group provides a unique platform for diverse functionalization reactions. While substitution reactions similar to those of benzene are possible, phenol’s hydroxyl group opens doors to reactions specific to alcohols as well. Phenol can undergo esterification to yield phenol esters, which find applications in perfumes and flavorings. Oxidation of phenol can yield quinones, which are essential intermediates in various biochemical pathways.
The hydroxyl group’s influence on phenol’s reactivity adds another layer of complexity to its chemistry, making it a versatile building block for a wide range of compounds.
In Conclusion: Two Aromatic Journeys
As our exploration of benzene and phenol draws to a close, we’re left with an appreciation for the intricate dance of atoms that gives rise to their distinctive properties and behaviors. From benzene’s resonance-driven stability to phenol’s hydroxyl-enhanced reactivity, these compounds teach us that a single change in structure can lead to a multitude of transformations in reactivity, applications, and even toxicity.
FAQs
Benzene features a hexagonal ring structure with alternating single and double bonds, showcasing its resonance-driven stability. In contrast, phenol’s structure includes a hydroxyl (-OH) group attached to the aromatic ring, enhancing its reactivity and influencing its properties.
Benzene prefers electrophilic aromatic substitution reactions due to its stability, whereas phenol participates in both substitution and addition reactions owing to the presence of the hydroxyl group. This unique reactivity sets them apart in their chemical behaviors.
Benzene serves as a precursor for polymers and various chemicals, playing a crucial role in industries such as petrochemicals and pharmaceuticals. Phenol finds applications in antiseptics, disinfectants, plastics, and resins, showcasing its versatility across medicine and manufacturing.
Benzene is classified as a carcinogen and poses health risks, contributing to air pollution and VOC emissions. In contrast, phenol is generally less hazardous, serving as a safer alternative, with a lower environmental impact.
Benzene has a lower boiling point due to London dispersion forces, while phenol exhibits a higher boiling point due to hydrogen bonding between molecules.
Absolutely! Benzene’s stability makes it suitable for substitution reactions, yielding diverse derivatives. Phenol’s hydroxyl group enables both substitution and alcohol-specific reactions, expanding its range of reactions and applications.
Benzene is colorless and has a characteristic pleasant aroma. Phenol, on the other hand, has a distinctive odor often described as medicinal or sharp.
Yes, due to its carcinogenic nature, benzene is subject to strict regulations to limit exposure in various industries. Phenol, while less hazardous, still requires proper handling due to its potential for irritation.
Both benzene and phenol are aromatic compounds, sharing a common hexagonal ring structure that defines their aromaticity. However, their differing functional groups and reactivity lead to their unique behaviors.
Explore detailed articles and references in reputable chemistry textbooks, academic journals, and online educational platforms to gain a deeper understanding of the contrasting attributes of benzene and phenol.
Read More:
Contents
- Differences Between Benzene and Phenol
- 1. Structural Foundation: A Tale of Rings and Groups
- 2. Reactivity and Chemical Behavior: Divergence Under the Surface
- 3. Applications: Where Chemistry Meets Utility
- 4. Physical Properties: The Dance of Intermolecular Forces
- 5. Toxicity and Environmental Impact: Weighing the Consequences
- 6. Derivatives and Functionalization: Crafting New Possibilities
- In Conclusion: Two Aromatic Journeys
- FAQs