| Feature | Aldehydes | Ketones |
|---|---|---|
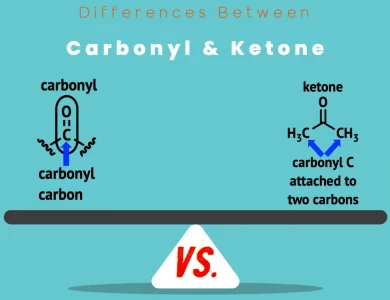
| Functional Group | Carbonyl group (C=O) bonded to a hydrogen (H) | Carbonyl group (C=O) bonded between two carbons |
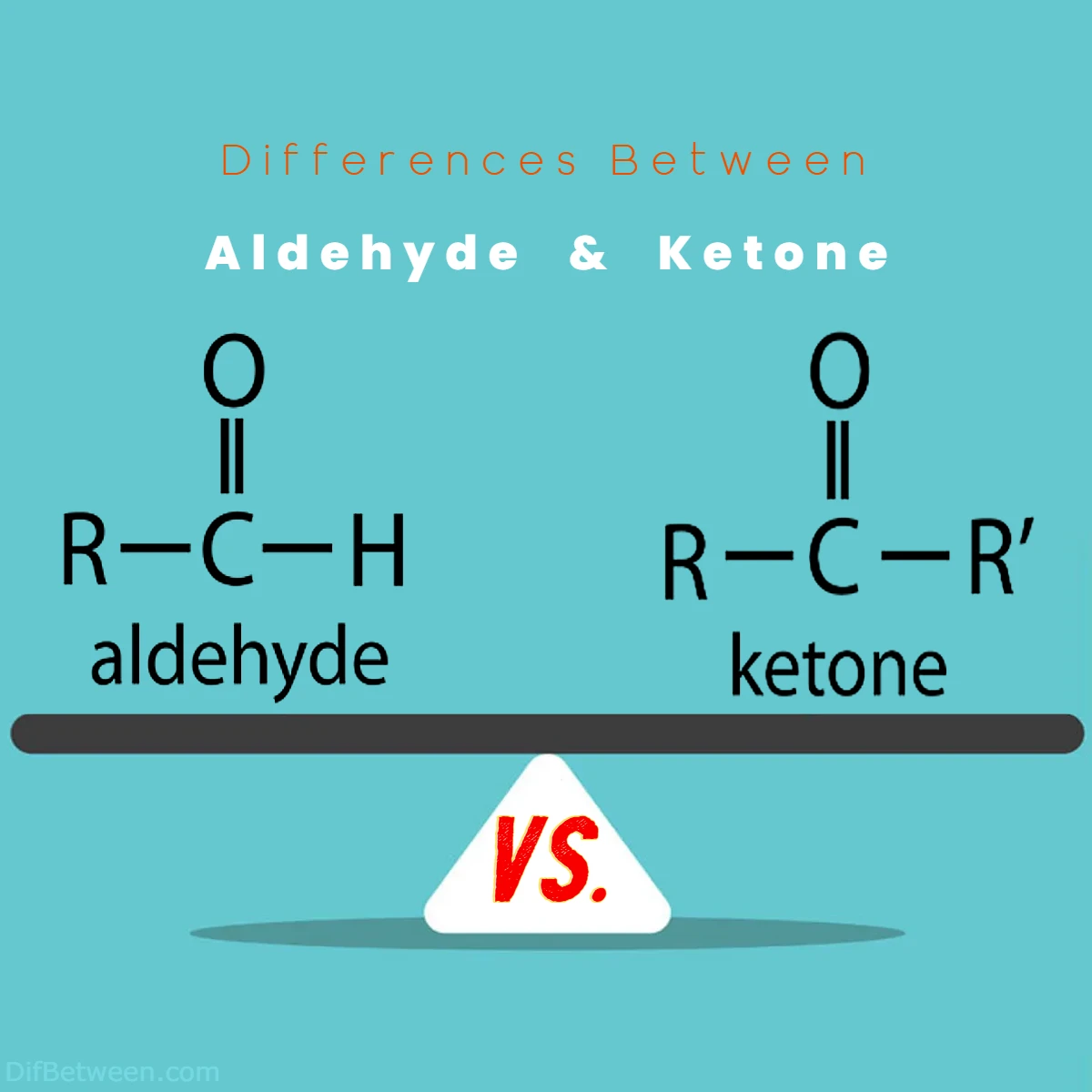
| General Formula | RCHO (R represents an alkyl or aryl group) | RCOR’ (R and R’ represent alkyl or aryl groups) |
| Oxidation Behavior | Easily oxidized to carboxylic acids | Not easily oxidized under mild conditions |
| Nucleophilic Reactions | Form hemiacetals and acetals | Form enols and enolates |
| Example Compound | Formaldehyde (HCHO) | Acetone (CH3COCH3) |
| Hydrogen Atom Presence | Hydrogen atom bonded directly to the carbonyl carbon | No hydrogen atom directly bonded to the carbonyl carbon |
| Oxidizing Agents | Easily oxidized by Tollens’ reagent, Fehling’s solution, etc. | Not readily oxidized under mild conditions |
| Nucleophilic Addition | React with compounds containing active hydrogen atoms | Engage in nucleophilic addition reactions |
| Tautomeric Equilibrium | Less likely to exhibit tautomeric equilibrium due to the presence of the hydrogen atom | More likely to exhibit tautomeric equilibrium and form enols |
| Applications | Fragrances, flavor compounds, preservation | Solvents, pharmaceutical synthesis, nail polish removers |
| Significance in Industries | Catalysts, intermediates, fine chemicals | Pharmaceutical synthesis, chiral compounds, solvent applications |
| Biochemical Roles | Participate in glycosidic bond formation, enzymatic reactions | Involvement in metabolic pathways, cellular processes |
| Computational Insights | Computational studies less focused on aldehydes due to reactivity | Computational studies aid in understanding ketone behavior |
| Materials Science | Limited applications in materials science | Serve as potential building blocks for diverse materials |
| Research Focus | Focus on reactivity, oxidation, and nucleophilic reactions | Focus on nucleophilic addition, equilibrium dynamics |
| Future Horizons | Potential for innovative synthetic methods, green chemistry | Applications in drug synthesis, nanotechnology, materials science |
The remarkable realm of aldehydes and ketones. These two unique classes of molecules are more than just chemical names you might have come across in textbooks; they are the architects of fragrance, the essence of flavor, and the silent players in numerous reactions that shape our world. So, buckle up as we traverse through the intricate landscapes of molecular structures, delve into the art of reactivity, and uncover the real-world applications that showcase the magic hidden within these compounds.
Differences Between Aldehyde and Ketone
The main differences between aldehyde and ketone lie in their molecular structures and reactivity. Aldehydes feature a carbonyl group (C=O) bonded to a hydrogen atom, while ketones possess a carbonyl group positioned between two carbon atoms. Aldehydes are easily oxidized to carboxylic acids, whereas ketones are not as readily oxidized. Furthermore, aldehydes engage in nucleophilic addition reactions, forming hemiacetals and acetals, while ketones partake in nucleophilic addition reactions, leading to the formation of enols and enolates. These distinctions in structure and behavior define their roles in various applications, from fragrances and flavors to pharmaceutical synthesis and materials science.
1. Unveiling the Molecular Identities
A. Aldehydes: The First Glimpse into their Nature
Let’s begin our journey with aldehydes. These compounds boast a distinctive structural feature that gives them a unique identity within the realm of organic molecules. At the heart of every aldehyde lies a functional group known as the aldehyde group, characterized by a carbonyl carbon atom (C=O) bonded to a hydrogen atom (H). This particular arrangement imparts aldehydes with a charming aura of reactivity and significance.
Aldehydes draw their name from the Latin term “alcohol dehydrogenatus,” which translates to “alcohol deprived of hydrogen.” This etymology is a nod to the fact that aldehydes are often derived from alcohols through a process that involves removing two hydrogen atoms—one from the hydroxyl group (-OH) of the alcohol and another from a neighboring carbon—to create the characteristic carbonyl group.
Table 1: A Glimpse at Aldehydes
| Feature | Aldehydes |
|---|---|
| Functional Group | Carbonyl group (C=O) bonded to a hydrogen (H) |
| General Formula | RCHO (R represents an alkyl or aryl group) |
| Example Compound | Formaldehyde (HCHO) |
B. Ketones: The Alluring Cousins
As we venture further into our exploration, we encounter ketones—a group of compounds that shares some similarities with aldehydes, yet possesses its own distinct characteristics. The hallmark of a ketone lies in its carbonyl group (C=O) that sits gracefully between two carbon atoms. Unlike aldehydes, ketones do not possess a hydrogen atom bonded directly to the carbonyl carbon.
The term “ketone” finds its roots in the German word “keton,” meaning acetone. Acetone, a simple and well-known ketone, was initially used as the representative compound for this functional group. Ketones often emerge as products of oxidation reactions, a transformation that adds an oxygen atom to a secondary alcohol’s carbon atom, creating the mesmerizing carbonyl group.
Table 2: A Glimpse at Ketones
| Feature | Ketones |
|---|---|
| Functional Group | Carbonyl group (C=O) positioned between carbons |
| General Formula | RCOR’ (R and R’ represent alkyl or aryl groups) |
| Example Compound | Acetone (CH3COCH3) |
A. Aldehydes: Reactivity Unveiled
Ah, reactivity—the heart of organic chemistry. Aldehydes, with their unique structure, exhibit intriguing behaviors that have captured the attention of chemists for centuries. One of the most iconic reactions involving aldehydes is their tendency to undergo oxidation. When an aldehyde encounters an oxidizing agent, such as Tollens’ reagent or Fehling’s solution, it undergoes transformation into a carboxylic acid.
Aldehydes also possess a remarkable ability to partake in nucleophilic addition reactions, particularly with compounds containing active hydrogen atoms. This reactivity forms the basis for the formation of hemiacetals and acetals, essential intermediates in various biochemical processes.
B. Ketones: The Spectacle of Reactivity
As we shift our focus to ketones, a different realm of reactivity unfolds. Ketones, lacking the active hydrogen of aldehydes, exhibit distinctive behaviors in their reactions. Oxidation reactions, which are the trademark of aldehydes, do not occur with ketones under mild conditions. This is due to the absence of the easily oxidizable hydrogen atom.
However, ketones shine in the arena of nucleophilic addition reactions. They readily engage with nucleophiles, resulting in the formation of intriguing products. The formation of enols and enolates, which serve as key intermediates in various synthetic transformations, is a remarkable example of ketone reactivity.
3. Real-world Applications and Significance
A. Aldehydes: Where We Encounter Them
Aldehydes are not mere abstract entities confined to chemistry textbooks. They play essential roles in various aspects of our lives. Let’s take a moment to appreciate the significance of aldehydes in our daily experiences.
- Fragrance and Flavor: The captivating scents and flavors that delight our senses often owe their existence to aldehydes. Perfumers and flavor chemists harness the unique aromatic properties of aldehydes to craft an array of scents and tastes.
- Preservation: Aldehydes find their place in the realm of preservation. Formaldehyde, for instance, is a potent preservative used to prevent the decay of biological specimens.
B. Ketones: Their Presence in the Real World
Just as aldehydes leave their mark, ketones too have made their way into various facets of our lives. Let’s explore where we might come across ketones beyond the laboratory.
- Solvent and Solubility: Ketones, such as acetone, serve as versatile solvents in laboratories and industries. Their ability to dissolve a wide range of compounds makes them invaluable in various processes.
- Nail Polish Removers: Ever wondered how nail polish vanishes so effortlessly? Ketone-based nail polish removers are the unsung heroes that make it happen. Acetone, with its remarkable solvent properties, effortlessly removes colorful coatings from nails.
4. Distinguishing Features: A Side-by-Side Comparison
Let’s take a moment to summarize the key distinctions between aldehydes and ketones, placing their defining features side by side.
Table 3: Side-by-Side Comparison of Aldehydes and Ketones
| Feature | Aldehydes | Ketones |
|---|---|---|
| Carbonyl Group Arrangement | C=O bonded to H | C=O bonded between two carbons |
| Oxidation Behavior | Easily oxidized to carboxylic acids | Not easily oxidized under mild conditions |
| Nucleophilic Reactions | Form hemiacetals and acetals | Form enols and enolates |
| Example Compound | Formaldehyde (HCHO) | Acetone (CH3COCH3) |
5. The Delicate Balance of Reactivity
A. Aldehydes: The Dance of Oxidation and Addition
Aldehydes possess a unique reactivity that stems from the interplay of their functional group and hydrogen atom. This reactivity is beautifully showcased in their oxidation reactions. Aldehydes readily give up their hydrogen atom to oxidizing agents, transforming into carboxylic acids—a reaction that sets them apart from ketones. The transformation of an aldehyde into a carboxylic acid involves the introduction of an oxygen atom into the molecule, accompanied by a shift in oxidation state.
But that’s not all! Aldehydes also engage in captivating nucleophilic addition reactions. The presence of the reactive hydrogen atom attached to the carbonyl carbon makes them susceptible to attack by nucleophiles, resulting in the formation of hemiacetals and acetals. These reactions are not only important in organic synthesis but also play vital roles in biological processes, such as glycosidic bond formation in carbohydrates.
B. Ketones: Navigating the Realm of Nucleophiles
Ketones, on the other hand, follow a different reactivity path, one marked by their lack of an active hydrogen atom. While they do not undergo oxidation reactions as readily as aldehydes, their interactions with nucleophiles are a spectacle to behold. The carbonyl group in ketones acts as a magnet for nucleophiles, drawing them in for addition reactions.
An intriguing example of ketone reactivity lies in the formation of enols and enolates. Under certain conditions, ketones can tango between their keto (carbonyl) and enol (carbon-carbon double bond with an attached hydroxyl group) forms. This tautomeric equilibrium opens the door to a variety of transformations, making ketones indispensable in synthetic endeavors.
6. Paving the Way in Industries
A. Aldehydes: Catalysts and Precursors
Aldehydes find themselves as catalysts and precursors in various industrial processes. Formaldehyde, a simple aldehyde, acts as a building block for the production of numerous resins, such as urea-formaldehyde and phenol-formaldehyde resins. These resins serve as adhesives, binders, and coatings, playing a pivotal role in the manufacturing of wood products and composite materials.
Moreover, aldehydes contribute to the field of fine chemicals, where they serve as intermediates for the synthesis of pharmaceuticals, agrochemicals, and flavor compounds. The delicate balance of their reactivity makes them valuable tools for chemists seeking to create complex molecules.
B. Ketones: Synthesis and Pharmaceuticals
Ketones, with their diverse reactivity and structural versatility, are treasured in the pharmaceutical and agrochemical industries. Medicinal chemists harness the power of ketones in synthesis, as they can be readily transformed into a myriad of functional groups. The availability of synthetic routes involving ketones empowers the creation of drug candidates with desired properties.
Ketones also play a vital role in the realm of asymmetric synthesis, where chemists aim to create molecules with specific spatial arrangements. Chiral ketones, which possess a distinct handedness, serve as starting materials in the synthesis of chiral drugs, enabling the production of single enantiomer products with enhanced pharmacological activity and reduced side effects.
7. Mastering the Art of Distinction
A. Aldehydes: A Final Look
As we near the culmination of our exploration, it’s worth noting that aldehydes stand as versatile compounds that effortlessly transition between reactivity and applications. The combination of their distinctive carbonyl group and reactive hydrogen atom bestows them with a charm that chemists and industries find irresistible.
B. Ketones: The Grand Finale
In our grand finale, we recognize ketones as formidable contenders in the world of organic chemistry. Their absence of an active hydrogen atom does not hinder their reactivity but rather propels them into a world of nucleophilic encounters, equilibrium shifts, and synthetic possibilities.
8. Further Exploration and Future Horizons
As we conclude our detailed exploration of aldehydes and ketones, we find ourselves at the threshold of even more exciting possibilities. The world of organic chemistry is a vast landscape, constantly evolving with new discoveries and applications. Let’s take a moment to consider the avenues for further exploration and the potential future horizons these compounds might unlock.
A. Cutting-edge Research
The realm of aldehydes and ketones continues to be a hotbed of cutting-edge research. Chemists are delving deeper into the reactivity of these compounds, uncovering new mechanisms and developing novel synthetic methods. Advances in catalysis, green chemistry, and sustainable synthesis are shaping the way we approach the production of valuable molecules.
B. Biochemical Insights
Aldehydes and ketones also play pivotal roles in the realm of biochemistry. Their involvement in metabolic pathways, enzymatic reactions, and signal transduction processes highlights their significance in understanding cellular processes. Ongoing research in this area could lead to breakthroughs in drug development, disease treatment, and personalized medicine.
C. Nanotechnology and Materials Science
The marriage of chemistry with materials science is a realm ripe for exploration. Aldehydes and ketones, with their ability to form versatile linkages, could serve as building blocks for novel materials with tailored properties. From biocompatible polymers to functional coatings, the versatility of these compounds opens up exciting possibilities in nanotechnology.
D. Computational Insights
The synergy between experimental research and computational chemistry is a burgeoning field. Computer simulations and quantum calculations provide insights into the behavior of aldehydes and ketones at the molecular level. This computational approach aids in predicting reactivity, understanding reaction mechanisms, and designing novel molecules with desired properties.
FAQs
The primary distinction lies in their functional groups and hydrogen atom arrangement. Aldehydes have a carbonyl group (C=O) bonded to a hydrogen atom, whereas ketones feature a carbonyl group situated between two carbon atoms.
Aldehydes are easily oxidized to form carboxylic acids, making them vulnerable to oxidizing agents. In contrast, ketones are not as readily oxidized under mild conditions due to the absence of an active hydrogen atom.
Indeed, they do. Aldehydes undergo nucleophilic addition reactions with compounds containing active hydrogen atoms, resulting in the formation of hemiacetals and acetals. Ketones engage in nucleophilic addition reactions as well, leading to the creation of enols and enolates.
Aldehydes are renowned for their roles in fragrances, flavors, and preservation (e.g., formaldehyde). Ketones find applications as solvents, pharmaceutical intermediates, and even nail polish removers (e.g., acetone).
Aldehydes serve as catalysts, intermediates, and building blocks in various industrial processes, including the synthesis of resins and fine chemicals. Ketones play a crucial role in pharmaceutical synthesis, chiral compound production, and materials science applications.
Yes, aldehydes tend to exhibit fewer tautomeric equilibria due to the presence of the hydrogen atom. Ketones, however, are more likely to undergo tautomeric shifts between their keto and enol forms.
Research in this field is dynamic, with a focus on sustainable synthesis, computational insights, and applications in nanotechnology. Both compounds continue to inspire advancements in chemistry, materials science, and various interdisciplinary areas.
Aldehydes and ketones serve as fundamental building blocks, influencing reactivity pathways, mechanisms, and the creation of complex molecules. Their distinct properties contribute to the rich tapestry of organic chemistry and its practical applications.
While distinct, aldehydes and ketones share a common carbonyl group, highlighting their relationship within the broader class of carbonyl compounds. Their nuanced differences make them intriguing subjects of study and exploration.
To dive deeper into the world of aldehydes and ketones, read our comprehensive blog titled “Differences Between Aldehyde vs Ketone.” This detailed exploration unveils the captivating traits, reactivity, and applications of these intriguing organic compounds.
Read More:
Contents
- Differences Between Aldehyde and Ketone
- 1. Unveiling the Molecular Identities
- 2. Navigating Reactivity: Aldehydes vs. Ketones
- 3. Real-world Applications and Significance
- 4. Distinguishing Features: A Side-by-Side Comparison
- 5. The Delicate Balance of Reactivity
- 6. Paving the Way in Industries
- 7. Mastering the Art of Distinction
- 8. Further Exploration and Future Horizons
- FAQs