| Aspect | Aldehyde | Formaldehyde |
|---|---|---|
| Chemical Structure | Contains a carbonyl group (C=O) bonded to a carbon and a hydrogen atom. | Contains a carbonyl group (H-C=O) bonded to hydrogen and oxygen atoms. |
| Physical State | Can exist as both liquids and gases at room temperature. | Exists as a colorless, pungent-smelling gas at room temperature. |
| Odor | Varies in odor, from fruity to sharp, depending on the specific compound. | Has a distinct pungent and strong odor. |
| Boiling Point | Boiling points generally higher than those of corresponding alcohols. | Boiling point is relatively low due to its small molecular size. |
| Reactivity | Participates in nucleophilic addition and oxidation reactions. | Reacts in nucleophilic addition reactions and can crosslink with proteins. |
| Applications | Used in perfumery, flavoring, pharmaceuticals, and organic synthesis. | Employed as a disinfectant, preservative, and in the production of materials. |
| Biochemical Role | Plays a role in metabolism and signaling pathways within living organisms. | Not a significant participant in biological processes. |
| Polymer Chemistry | Limited role in polymer chemistry compared to other functional groups. | Essential for the production of formaldehyde-based resins used in construction and materials manufacturing. |
| Health Impact | Generally safe in small amounts but can cause irritation or allergies in some individuals. | Prolonged exposure can lead to respiratory irritation, sensitization, and health issues. |
| Environmental Impact | Can contribute to air pollution when emitted as pollutants. | Formaldehyde emissions can contribute to air pollution and ground-level ozone formation. |
| Analytical Techniques | Detected and quantified using gas chromatography, HPLC, and colorimetric assays. | Analyzed using similar techniques as aldehydes, with emphasis on formaldehyde-specific methods. |
| Derivatives | Can form various derivatives through reactions like aldol reactions and reduction to alcohols. | Participates in similar reactions as aldehydes, leading to a variety of derivatives. |
| Formaldehyde-Free Alternatives | Not applicable as aldehydes themselves are not always used as standalone products. | Ongoing research to develop alternatives for applications involving formaldehyde. |
| Emerging Research | Focus on catalytic transformations and biocatalysis for selective modifications. | Research aims to develop greener and safer methods for formaldehyde-related processes. |
| Educational Outreach | Educational campaigns focus on raising awareness about aldehyde presence in products. | Efforts target promoting awareness of formaldehyde exposure sources and safer practices. |
Imagine walking through an orchard, surrounded by the enchanting fragrance of fruits and flowers. Ever wondered what gives those scents their distinctive charm? That’s where aldehydes step onto the stage. These compounds play a role in everything from the tantalizing flavors in your favorite foods to the delightful aromas of perfumes. But hold on to your hats because we’re about to meet the enigmatic formaldehyde. Often associated with preservation and chemistry labs, formaldehyde has its own set of surprises up its sleeve. It’s not just a pungent gas; it’s a key player in everything from disinfection to crafting durable materials.
Differences Between Aldehyde and Formaldehyde
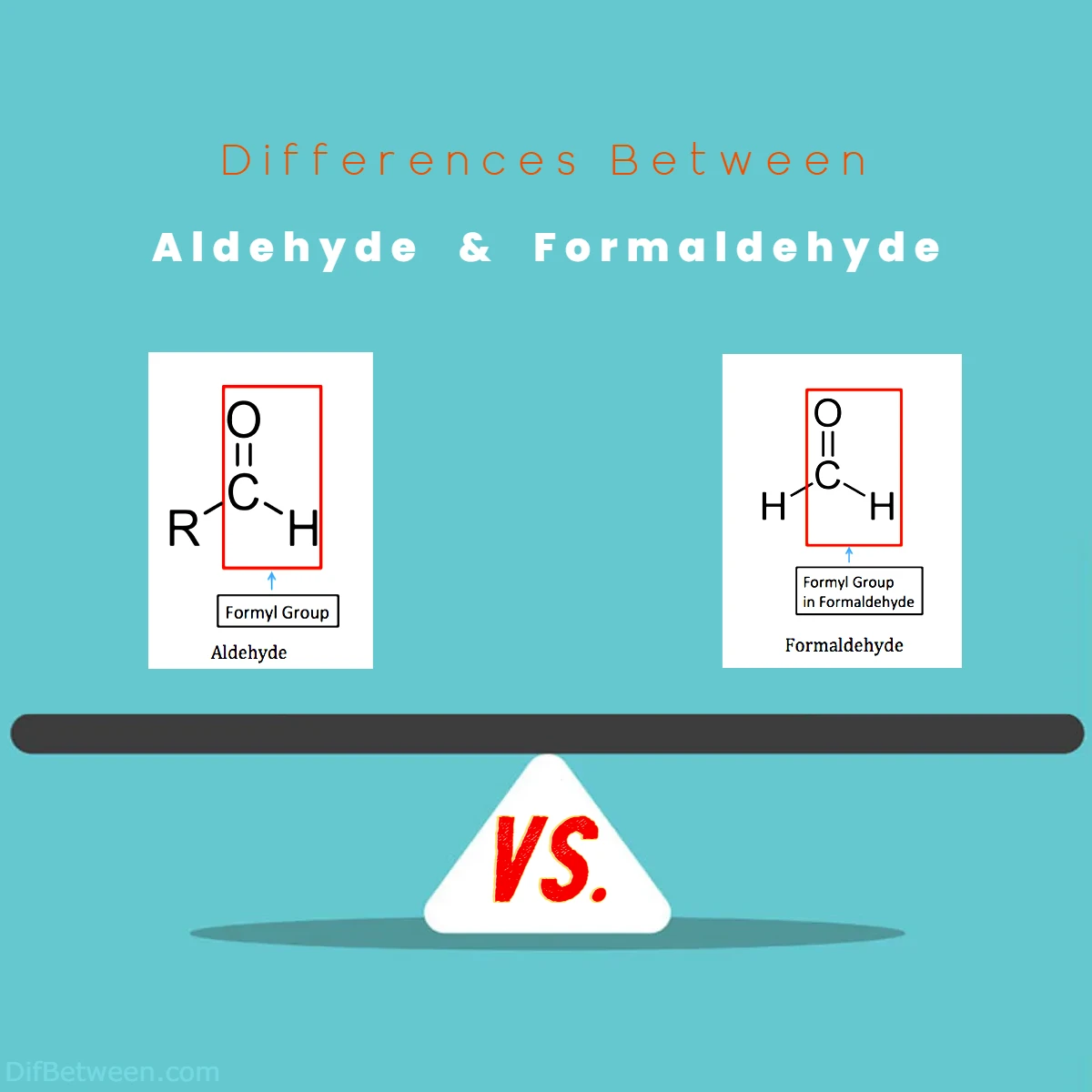
The main differences between aldehyde and formaldehyde lie in their chemical structures and properties. Aldehydes are organic compounds characterized by a carbonyl group (C=O) bonded to a carbon and a hydrogen atom. They are versatile molecules with distinct odors, found in perfumes, flavors, and industrial processes. On the other hand, formaldehyde is a specific aldehyde with a carbonyl group (H-C=O) bonded to hydrogen and oxygen atoms. It exists as a pungent gas and is known for its preservation, disinfection, and industrial applications. While both compounds share common features, their unique structures and uses set them apart in the world of chemistry.
1. The Basics: Aldehyde and Formaldehyde Unveiled
Aldehyde: Picture a charming house with a front porch, and right above the porch, you’ll spot a ‘C’ shape suspended in mid-air. Now, that ‘C’ shape represents the carbonyl group (C=O) that characterizes an aldehyde. Aldehydes belong to the family of organic compounds, where carbon and hydrogen join hands to create molecules that play crucial roles in various biological and chemical processes. These compounds are found in both natural and synthetic forms, adding their distinctive touch to the aroma of essential oils, the taste of certain foods, and even in pharmaceuticals.
Formaldehyde: Ah, formaldehyde, the compound that often conjures up images of chemistry labs and preservation techniques. This particular member of the aldehyde family is a colorless gas with a pungent odor, and it boasts a structure similar to its aldehyde siblings. The ‘C’ shape is once again present, holding hands with oxygen to create the carbonyl group (HCHO). Formaldehyde’s fame, or rather notoriety, arises from its widespread use as a preservative, disinfectant, and even in the manufacture of various materials.
2. A Closer Look at Structures
Now that we’ve had a charming introduction to aldehydes and formaldehyde, let’s take a closer look at their structures. While both compounds share a common feature—the carbonyl group—they also exhibit distinctive arrangements that contribute to their unique properties and behaviors.
Aldehyde Structure: Imagine a carbon atom as the heart of the molecule, surrounded by hydrogen atoms on one side and the precious carbonyl group (C=O) on the other. This structure gives aldehydes their characteristic reactivity, making them a versatile bunch in the world of chemical reactions.
Formaldehyde Structure: Formaldehyde, being an aldehyde, showcases a similar arrangement at its core. A single carbon atom stands tall, bonded to hydrogen atoms on one end, and the carbonyl group (HCHO) on the other. This structure gives formaldehyde its remarkable ability to react with other compounds, which is why it’s often employed as a crosslinking agent and preservative.
3. Diverse Properties: Aldehyde vs. Formaldehyde
Now that we’ve grasped the structural intricacies, let’s shift our focus to the properties that set aldehydes and formaldehyde apart. These properties pave the way for their distinct roles in various applications.
Physical Properties of Aldehydes: Aldehydes exhibit a wide range of physical properties, owing to the varying lengths of their carbon chains and the nature of substituents attached to the carbonyl group. They often have lower boiling points compared to alcohols and carboxylic acids of similar molecular weight. Aldehydes like formaldehyde are volatile due to their low molecular weights, making them readily vaporize at room temperature. The presence of the carbonyl group gives aldehydes their characteristic odor, which can range from fruity and pleasant to intensely sharp.
Chemical Properties of Aldehydes: Aldehydes are no wallflowers when it comes to chemical reactions. Thanks to the electronegativity of oxygen in the carbonyl group, aldehydes tend to be electron-deficient, making them susceptible to nucleophilic attack. This reactivity enables them to partake in reactions like nucleophilic addition and oxidation. For instance, aldehydes can be easily oxidized to carboxylic acids.
Physical Properties of Formaldehyde: Formaldehyde, as a gas, presents unique physical properties. It has a pungent odor that is detectable even at low concentrations, which is why it’s often recognized as a warning sign for potential exposure. Formaldehyde gas is soluble in water, and its solutions, known as formalin, find use in preserving biological specimens. Due to its small molecular size, formaldehyde molecules are easily vaporized, allowing it to be used as a disinfectant by gaseous fumigation.
Chemical Properties of Formaldehyde: Formaldehyde’s chemical behavior is influenced by its reactivity as an aldehyde. It readily undergoes reactions such as nucleophilic addition, forming derivatives that have diverse applications. One of its most important reactions is the ability to crosslink with proteins, which is why it’s used for preserving biological samples and creating durable materials like particleboard.
4. Applications in the Real World
It’s time to explore the practical realm where aldehydes and formaldehyde shine, each contributing its unique touch to various applications across industries.
Aldehyde Applications: Aldehydes find their way into numerous sectors, leaving their aromatic imprint. Perfumery owes a lot to aldehydes, as they’re responsible for adding delightful scents to fragrances. In the world of flavors, aldehydes lend their essence to foods, enhancing taste profiles. Some aldehydes, like acetaldehyde, are utilized in the production of plastics and synthetic resins. Moreover, aldehydes are pivotal intermediates in organic synthesis, playing a crucial role in the creation of more complex molecules.
Formaldehyde Applications: Formaldehyde’s reputation might precede it, but its uses extend far beyond preservation. As a disinfectant, formaldehyde finds its way into laboratories and healthcare facilities, safeguarding against harmful microorganisms. The production of textiles and cosmetics benefits from formaldehyde’s role as a crosslinking agent. Additionally, formaldehyde-based resins are integral to the manufacturing of plywood, furniture, and even automobile parts. The medical field relies on formaldehyde for embalming and as an ingredient in vaccines.
5. Safety Considerations and Environmental Impact
Now, as responsible explorers, we must address the safety considerations and environmental impact associated with these compounds.
Aldehyde Safety and Environmental Impact: While many aldehydes are naturally occurring and generally safe when encountered in small amounts (such as in foods and fragrances), some can cause irritation, allergies, or toxicity upon prolonged exposure. It’s essential to handle aldehydes cautiously in laboratory settings. In terms of environmental impact, certain aldehydes can be emitted as pollutants in the air, contributing to air quality concerns.
Formaldehyde Safety and Environmental Impact: Formaldehyde, due to its pungent nature, can cause respiratory irritation and sensitization in humans. Prolonged exposure to high concentrations can lead to more severe health issues. In terms of the environment, formaldehyde emissions can contribute to air pollution. Additionally, formaldehyde is considered a volatile organic compound (VOC), which can contribute to the formation of ground-level ozone, a component of smog.
6. The Role of Aldehydes in Biochemistry
Now, let’s dive deeper into the realm of biochemistry and uncover how aldehydes play an essential role in various biological processes.
Aldehydes in Metabolism: Aldehydes are integral to metabolic pathways within living organisms. For instance, in glycolysis—the process of breaking down glucose for energy—glyceraldehyde-3-phosphate, an aldehyde derivative, is a key intermediate. Aldehydes are also involved in the biosynthesis of fatty acids, where they act as building blocks for creating lipid molecules.
Aldehyde Signaling: Aldehydes can also serve as signaling molecules within cells. One notable example is retinal, an aldehyde derivative of vitamin A. Retinal is crucial for vision as it binds to opsins in photoreceptor cells, initiating the process of signal transduction that allows us to perceive light.
7. Formaldehyde in Polymer Chemistry
While formaldehyde is often associated with preservation and disinfection, it also boasts a significant presence in the world of polymer chemistry.
Formaldehyde Resins: Formaldehyde is a key ingredient in the production of various types of resins, such as urea-formaldehyde, melamine-formaldehyde, and phenol-formaldehyde resins. These resins are used in the manufacturing of plywood, particleboard, and other composite materials. They provide structural stability, durability, and resistance to moisture and heat, making them essential components in the construction and furniture industries.
Crosslinking in Polymers: Formaldehyde’s unique ability to crosslink with other molecules is harnessed in the creation of polymers. Crosslinking involves forming strong bonds between polymer chains, enhancing the material’s mechanical properties and stability. This process is crucial in the production of textiles, adhesives, and coatings, where formaldehyde-based resins improve the quality and performance of the end products.
8. Health and Safety Considerations
Ensuring the safety of individuals working with or exposed to aldehydes and formaldehyde is of paramount importance.
Aldehyde Exposure: While naturally occurring aldehydes in foods and fragrances are generally safe in small quantities, individuals with sensitivities or allergies should exercise caution. In laboratory settings, proper ventilation and personal protective equipment should be used to minimize exposure to potentially harmful aldehydes.
Formaldehyde Exposure: Formaldehyde exposure, especially in high concentrations, can lead to health issues such as respiratory irritation, allergies, and skin sensitization. In occupational settings where formaldehyde is used or produced, strict safety measures should be in place, including ventilation, protective clothing, and monitoring of air quality. Individuals with pre-existing respiratory conditions may be particularly vulnerable to formaldehyde exposure.
9. Environmental Impact and Regulation
The environmental impact of aldehydes and formaldehyde is a topic of concern, prompting regulatory measures to mitigate their effects.
Air Quality and Emissions: Certain aldehydes, including formaldehyde, can contribute to air pollution. Emissions from industrial processes, vehicle exhaust, and other sources can lead to the formation of smog and ground-level ozone, which have adverse effects on air quality and human health.
Regulatory Measures: Many countries have established regulations and standards for formaldehyde emissions, particularly in indoor environments where exposure can be significant. These regulations apply to products such as building materials, furniture, and textiles, aiming to limit formaldehyde emissions and protect human health.
10. Advancements and Future Prospects
As scientific research and technology continue to advance, new opportunities and challenges arise in the realm of aldehydes and formaldehyde.
Green Chemistry: Researchers are exploring greener and more sustainable methods for the production and utilization of aldehydes and formaldehyde derivatives. Green chemistry principles aim to minimize the environmental impact of chemical processes, leading to the development of more eco-friendly alternatives.
Healthcare and Materials Science: Advances in materials science are likely to lead to innovations in formaldehyde-based resins and polymers. Additionally, the medical field could benefit from improved preservation techniques that reduce formaldehyde exposure while maintaining the quality of biological specimens.
11. Aldehyde and Formaldehyde: Analytical Techniques
The identification and quantification of aldehydes and formaldehyde are vital for quality control, research, and regulatory purposes. Several analytical techniques are employed to detect and measure these compounds accurately.
Gas Chromatography (GC): Gas chromatography is a widely used technique to separate and quantify aldehydes and formaldehyde in complex mixtures. Samples are vaporized and injected into a chromatograph, where they are separated based on their interaction with a stationary phase. Detector responses provide quantitative data about the presence and concentration of specific aldehydes.
High-Performance Liquid Chromatography (HPLC): HPLC is another valuable method for analyzing aldehydes and formaldehyde. In this technique, liquid samples are pumped through a column containing a stationary phase, separating compounds based on their affinity for the stationary phase. UV or fluorescence detectors help quantify the analytes.
Colorimetric Methods: Colorimetric assays are often employed for rapid and qualitative analysis of formaldehyde. These assays involve chemical reactions that produce color changes in the presence of formaldehyde, enabling visual detection or quantification through spectrophotometry.
Spectroscopic Techniques: Techniques such as infrared (IR) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy are used for structural characterization of aldehydes and formaldehyde. These methods provide information about molecular vibrations and chemical shifts, aiding in compound identification.
12. Aldehyde Derivatives and Functional Groups
Aldehydes can undergo various reactions to form derivatives and functional groups, enhancing their versatility in organic synthesis and applications.
Aldol Reactions: Aldol reactions involve the condensation of two carbonyl compounds—an aldehyde and a ketone—in the presence of a base. The resulting product is a β-hydroxyaldehyde, which can then be further modified to yield various compounds.
Reduction to Alcohols: Aldehydes can be reduced to alcohols using reducing agents such as sodium borohydride or lithium aluminum hydride. This conversion is a common step in the synthesis of pharmaceuticals and fine chemicals.
Oxidation to Carboxylic Acids: Under suitable conditions, aldehydes can be oxidized to carboxylic acids using oxidizing agents like potassium permanganate or chromic acid. This reaction pathway is essential in the metabolism of certain compounds in living organisms.
13. Formaldehyde-Free Alternatives
Given concerns about formaldehyde’s health and environmental impacts, there’s a growing interest in developing formaldehyde-free alternatives for various applications.
Preservation: In preservation and disinfection, researchers are exploring alternative methods that do not rely on formaldehyde. These methods include using non-formaldehyde-based biocides, UV radiation, and advanced oxidation processes.
Wood-Based Products: The construction and furniture industries are seeking formaldehyde-free alternatives for adhesives and resins. Water-based adhesives and bio-based resins are being developed to reduce formaldehyde emissions from composite wood products.
14. Emerging Research and Innovations
As the field of chemistry continues to evolve, emerging research areas and innovations hold the promise of uncovering new insights and applications for aldehydes and formaldehyde.
Catalytic Transformations: Researchers are exploring catalytic processes to selectively modify aldehydes and formaldehyde derivatives. Catalysis allows for more sustainable and efficient chemical transformations, reducing the need for harsh reagents.
Biocatalysis: Enzymes and biocatalysts are being harnessed to perform reactions involving aldehydes and formaldehyde derivatives. These enzymatic processes are environmentally friendly and offer high selectivity in producing desired products.
15. Educational Outreach and Public Awareness
Enhancing public awareness about the properties, uses, and potential risks associated with aldehydes and formaldehyde is crucial for informed decision-making and responsible usage.
Educational Campaigns: Educational initiatives can empower individuals to understand the presence of aldehydes in everyday products and make informed choices. These campaigns can raise awareness about formaldehyde exposure sources and encourage safer practices.
Lab Safety Training: Proper lab safety training ensures that individuals working with aldehydes and formaldehyde are equipped with the knowledge and skills to handle these compounds safely. Training programs emphasize the importance of protective equipment, ventilation, and proper disposal of waste.
FAQs
Aldehyde is a class of organic compounds containing a carbonyl group (C=O) bonded to a carbon and a hydrogen atom. Formaldehyde, on the other hand, is a specific aldehyde with a carbonyl group (H-C=O) bonded to hydrogen and oxygen atoms. While all formaldehydes are aldehydes, not all aldehydes are formaldehydes.
Aldehydes can vary in odor, ranging from fruity to sharp, and can exist as both liquids and gases at room temperature. In contrast, formaldehyde is a colorless gas with a strong, pungent odor, existing solely as a gas at room temperature.
Aldehydes find applications in perfumery, flavoring, pharmaceuticals, and organic synthesis. They contribute to the aroma of fragrances, enhance flavors in foods, and serve as intermediates in various chemical processes. Formaldehyde, with its preservation and disinfection properties, is used as a disinfectant, in the manufacture of materials, and for embalming.
Aldehyde exposure in small amounts from natural sources like foods and fragrances is generally safe. However, formaldehyde exposure, even at low concentrations, can lead to respiratory irritation, allergies, and sensitization. Both aldehydes and formaldehyde can contribute to air pollution, with formaldehyde emissions having additional implications due to its status as a volatile organic compound (VOC).
Yes, efforts are being made to develop formaldehyde-free alternatives in areas where formaldehyde is used, such as preservation and construction. These alternatives aim to reduce health and environmental risks associated with formaldehyde exposure.
The future holds exciting possibilities for advancements in catalytic transformations, biocatalysis, and greener methods involving these compounds. Research aims to harness their unique reactivity for sustainable applications in various industries.
To dive deeper into the captivating world of aldehyde and formaldehyde, continue reading our comprehensive blog “Differences Between Aldehyde vs Formaldehyde.” Discover their structural nuances, explore their roles in diverse applications, and gain insights into their impact on health and the environment. Get ready to be amazed by the intriguing tales that these compounds have to tell!
Read More:
Contents
- Differences Between Aldehyde and Formaldehyde
- 1. The Basics: Aldehyde and Formaldehyde Unveiled
- 2. A Closer Look at Structures
- 3. Diverse Properties: Aldehyde vs. Formaldehyde
- 4. Applications in the Real World
- 5. Safety Considerations and Environmental Impact
- 6. The Role of Aldehydes in Biochemistry
- 7. Formaldehyde in Polymer Chemistry
- 8. Health and Safety Considerations
- 9. Environmental Impact and Regulation
- 10. Advancements and Future Prospects
- 11. Aldehyde and Formaldehyde: Analytical Techniques
- 12. Aldehyde Derivatives and Functional Groups
- 13. Formaldehyde-Free Alternatives
- 14. Emerging Research and Innovations
- 15. Educational Outreach and Public Awareness
- FAQs