| Property | Carbonyl Compounds (General) | Ketones |
|---|---|---|
| Definition | A functional group containing a carbon atom doubly bonded to an oxygen atom (C=O). | A specific subgroup of carbonyl compounds where the C(=O) bond is between two carbon groups. |
| Structural Arrangement | Carbonyl group can be part of various functional groups like aldehydes, ketones, carboxylic acids, esters, and amides. | Carbonyl group situated between two carbon-containing groups. |
| Polarity | Generally polar due to the electronegativity difference between carbon and oxygen in the C=O bond. | Polar due to the electronegativity difference in the C=O bond. |
| Boiling Point | Generally higher compared to nonpolar compounds of similar size due to polarity. | Generally lower than carboxylic acids and aldehydes of similar size. |
| Melting Point | Generally higher compared to nonpolar compounds of similar size due to polarity. | Generally lower than carboxylic acids and aldehydes of similar size. |
| Solubility in Water | Often soluble due to hydrogen bonding between the carbonyl oxygen and water. | Often soluble due to hydrogen bonding with water, especially for smaller ketones. |
| Reactivity | Versatile reactivity, including nucleophilic addition, oxidation, reduction, condensation reactions, and tautomeric behavior. | Similar reactivity to carbonyl compounds, including nucleophilic addition, reduction, and condensation reactions. More resistant to oxidation. |
| Spectroscopic Features | Strong IR absorption band around 1700 cm^-1 due to carbonyl stretching vibration. | Strong IR absorption band around 1700 cm^-1 due to carbonyl stretching vibration. |
| Isomerism | Exhibits structural isomerism (e.g., position of carbonyl group) and tautomeric isomerism in some cases. | Exhibits structural isomerism based on the nature of the carbon groups attached to the carbonyl group. |
| Biological Significance | Found in biomolecules; aldehydes involved in metabolic pathways. | Ketones play a role in energy metabolism, especially during ketosis. |
| Environmental Impact | Some aldehydes and ketones can contribute to air pollution. | Environmental impact varies based on specific compounds and usage. |
| Safety Considerations | Some aldehydes are flammable and can be irritating. | Ketones like acetone are flammable and volatile. Precautions should be taken. |
| Applications | Broad applications, including pharmaceuticals, perfumery, food industry, and polymers. | Solvents, pharmaceutical intermediates, flavors, and fragrances. |
| Natural Occurrence | Found in various natural products, contributing to scents and flavors. | Produced naturally in the body during fasting or low carbohydrate intake. |
| Future Perspectives | Research focused on green chemistry, drug development, and metabolic health. | Continued exploration of ketosis and potential therapeutic applications. |
Carbonyl compounds, with their distinctive C=O bond, and ketones, a specific subset within this category, are like the “stars” of organic chemistry. They may seem subtle on the molecular stage, but their roles are anything but insignificant. From influencing the aroma of your morning coffee to playing pivotal roles in pharmaceutical breakthroughs, carbonyl compounds and ketones are essential players in the grand chemistry narrative.
Differences Between Carbonyl and Ketone
The main differences between carbonyl compounds and ketones lie in their structural arrangement and reactivity. Carbonyl compounds encompass a broader category, featuring a carbon atom double-bonded to an oxygen atom (C=O), and can be found in various functional groups like aldehydes, ketones, carboxylic acids, esters, and amides. On the other hand, ketones are a specific subgroup of carbonyl compounds where the C(=O) bond is situated between two carbon-containing groups. This structural distinction leads to differences in physical properties, such as boiling and melting points, and reactivity patterns, including their susceptibility to oxidation. Understanding these fundamental disparities is essential for anyone delving into the world of organic chemistry.
1. The Molecular Identities
Carbonyl Compounds
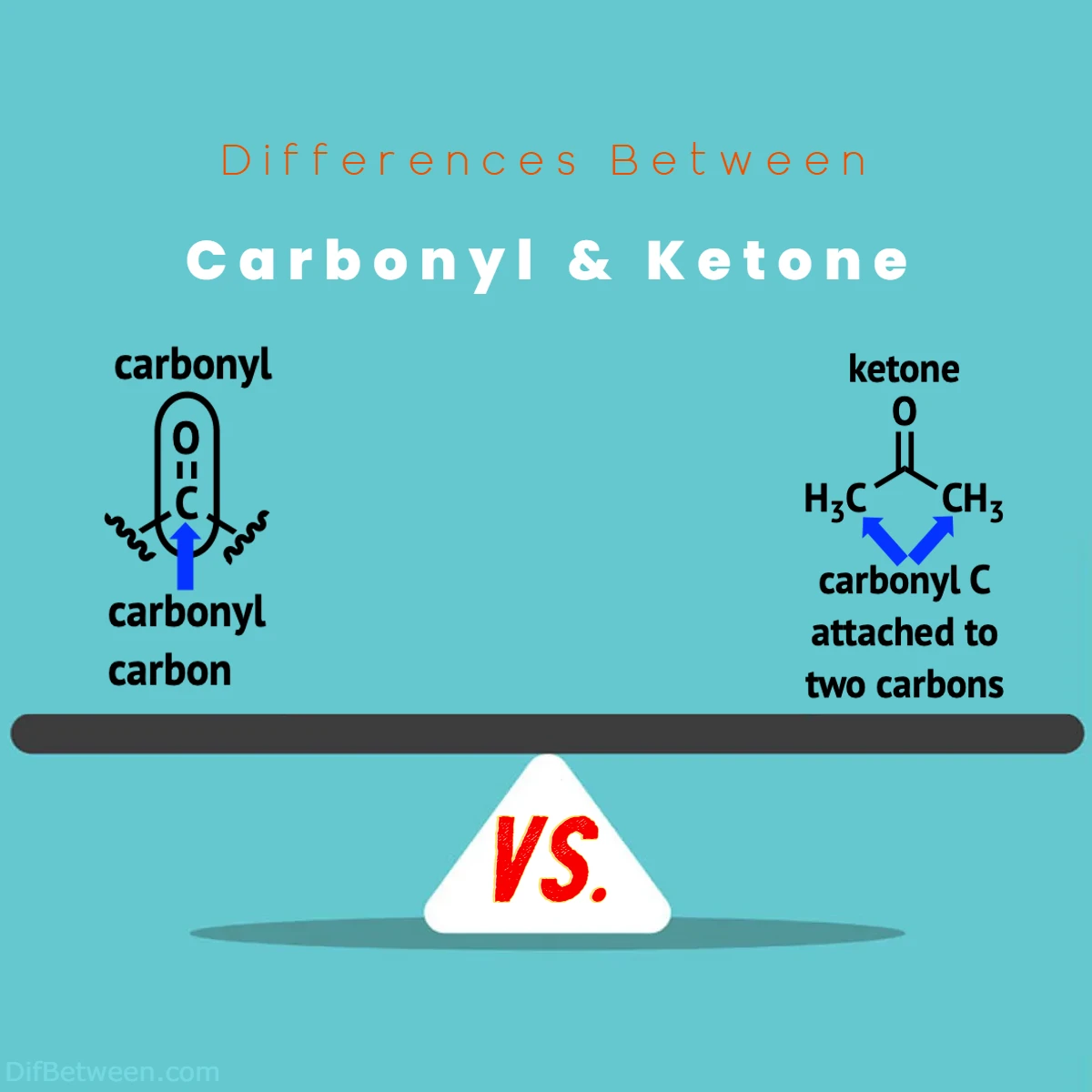
A carbonyl compound is a broad term that encompasses a functional group consisting of a carbon atom doubly bonded to an oxygen atom, represented as C=O. It’s worth noting that carbonyl groups can be found in various organic molecules, including aldehydes, ketones, carboxylic acids, esters, and amides. The distinguishing factor among these compounds lies in the surrounding atoms bonded to the carbonyl group.
In an aldehyde, the carbonyl group is situated at the end of a carbon chain, with one hydrogen atom and one alkyl or aryl group bonded to the carbon atom. For instance, formaldehyde is the simplest aldehyde with the structure H-CHO, where CHO represents the carbonyl group.
Ketones
On the other hand, ketones are a specific subset of carbonyl compounds. In ketones, the carbonyl group is positioned within the carbon chain, bonded to two alkyl or aryl groups. The general structure of a ketone can be represented as R-C(=O)-R’, where R and R’ denote different alkyl or aryl groups. Acetone, a commonly used solvent, is a classic example of a ketone with the structure CH3-C(=O)-CH3.
In essence, the primary difference between carbonyl compounds and ketones is their structural arrangement. While carbonyl compounds encompass a broader category with various functional groups containing the C=O bond, ketones are a distinct subgroup characterized by the C(=O) attachment between two carbon-containing groups.
2. Physical Properties
Carbonyl Compounds
Carbonyl compounds, being a diverse group, display a wide range of physical properties. However, some common characteristics are associated with the carbonyl functional group itself. One notable feature is polarity. The oxygen atom in the C=O bond is more electronegative than carbon, resulting in an unequal sharing of electrons. This creates a partial negative charge on the oxygen atom and a partial positive charge on the carbon atom, making the carbonyl group polar.
As a consequence of this polarity, carbonyl compounds often exhibit higher boiling points and melting points compared to nonpolar compounds of similar molecular weight. Additionally, the polarity of the carbonyl group imparts a significant influence on their solubility properties. Many carbonyl compounds, especially those with smaller carbon chains, are soluble in polar solvents like water due to the formation of hydrogen bonds between the oxygen of the carbonyl group and water molecules.
Ketones
Ketones, as a subset of carbonyl compounds, share some of these physical properties. They also tend to have higher boiling and melting points compared to nonpolar compounds of similar size due to the polarity of the carbonyl group. However, ketones generally have lower boiling points than carboxylic acids and aldehydes of comparable molecular weight because they lack the ability to form strong hydrogen bonds through the carbonyl oxygen.
Another noteworthy feature of ketones is their characteristic smell. Many ketones, particularly those with low molecular weights, possess distinctive odors. For instance, acetone is known for its sweet and somewhat pungent odor. This property makes ketones valuable in perfumery and flavor chemistry.
Let’s summarize the key physical differences between carbonyl compounds and ketones in a table:
| Property | Carbonyl Compounds | Ketones |
|---|---|---|
| Structure | C=O is part of various functional groups | C(=O) bonded between two carbon groups |
| Polarity | Polar due to the C=O bond | Polar due to the C=O bond |
| Boiling Point | Generally higher compared to nonpolar compounds of similar size | Generally lower than carboxylic acids and aldehydes of similar size |
| Melting Point | Generally higher compared to nonpolar compounds of similar size | Generally lower than carboxylic acids and aldehydes of similar size |
| Solubility in Water | Often soluble due to hydrogen bonding with water | Often soluble due to hydrogen bonding with water (for smaller ketones) |
3. Reactivity and Chemical Behavior
Carbonyl Compounds
Carbonyl compounds are known for their versatility in chemical reactions. The presence of the polar C=O bond renders them susceptible to nucleophilic and electrophilic attacks. Here are some key reactions and behaviors associated with carbonyl compounds:
1. Nucleophilic Addition: Carbonyl compounds readily undergo nucleophilic addition reactions. In these reactions, a nucleophile, attracted to the partially positive carbon in the carbonyl group, adds to the carbonyl carbon, breaking the π bond and forming two new σ bonds. This results in the formation of alcohol derivatives. Aldehydes and ketones, for instance, can be converted to alcohols through nucleophilic addition reactions.
2. Oxidation: Carbonyl compounds can be further oxidized to carboxylic acids through various oxidation reactions. For example, aldehydes can be oxidized to carboxylic acids using strong oxidizing agents like potassium permanganate (KMnO4) or dichromate (Cr2O7^2-).
3. Reduction: Carbonyl compounds can be selectively reduced to form alcohols. Common reducing agents include sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4).
4. Condensation Reactions: Carbonyl compounds are prone to undergo condensation reactions, leading to the formation of larger molecules. For instance, two molecules of an aldehyde or ketone can react to form a symmetric or asymmetric ketone, often with the elimination of water.
5. Tautomerism: Some carbonyl compounds exhibit tautomeric behavior, where the position of a hydrogen atom shifts within the molecule, resulting in a different isomeric form. For example, keto-enol tautomerism is observed in certain carbonyl compounds.
Ketones
Ketones share many of the reactivity traits observed in carbonyl compounds, owing to the presence of the carbonyl group. However, there are some nuances in their behavior:
1. Nucleophilic Addition: Ketones, like other carbonyl compounds, undergo nucleophilic addition reactions. In this context, ketones often react with nucleophiles to form alcohol derivatives.
2. Oxidation: Unlike aldehydes, ketones are relatively resistant to oxidation reactions under mild conditions. It generally requires harsh conditions and strong oxidizing agents to convert a ketone into a carboxylic acid.
3. Reduction: Ketones can be selectively reduced to form secondary alcohols, where the carbonyl group is replaced by a hydroxyl group.
4. Condensation Reactions: Ketones can participate in various condensation reactions, particularly in the synthesis of complex molecules in organic chemistry.
In summary, both carbonyl compounds and ketones exhibit similar reactivity patterns, with some distinctions in their susceptibility to oxidation and their specific behavior in certain reactions. Their versatility in organic synthesis makes them invaluable tools for chemists in various fields.
4. Practical Applications
Carbonyl Compounds
1. Perfumery and Fragrance Industry: Carbonyl compounds play a crucial role in creating the scents and fragrances we encounter in perfumes, colognes, and scented products. Aldehydes, in particular, are known for their aromatic contributions.
2. Pharmaceutical Industry: Carbonyl compounds are frequently used as intermediates in the synthesis of pharmaceuticals. They serve as building blocks for many drugs and are involved in key steps of drug development.
3. Food Industry: Some carbonyl compounds are natural flavoring agents found in fruits, vegetables, and spices. They are also used as food additives to enhance flavors and aromas in processed foods.
4. Polymer Industry: Carbonyl compounds are employed in the production of various polymers and plastics. For example, formaldehyde is a key component in the synthesis of phenolic resins.
Ketones
1. Solvents: Ketones like acetone and methyl ethyl ketone (MEK) are widely used as solvents in industrial applications. Acetone, in particular, is a versatile solvent used for cleaning, degreasing, and in the production of paints and coatings.
2. Pharmaceuticals: Ketones find applications in the pharmaceutical industry as starting materials for the synthesis of drug compounds. They can also be used as chiral auxiliaries in asymmetric synthesis.
3. Flavors and Fragrances: Certain ketones contribute to the aroma and taste of various foods and beverages. They are used as flavoring agents in the food industry.
4. Organic Synthesis: Ketones are essential intermediates in organic synthesis, allowing chemists to build complex molecules efficiently. They serve as precursors for a wide range of chemical reactions.
In essence, carbonyl compounds and ketones are ubiquitous in various industries and applications, from enhancing the flavors of our food to enabling the creation of life-saving pharmaceuticals. Their distinct properties and reactivity make them indispensable in the world of chemistry and beyond.
5. Spectroscopic Analysis
Carbonyl Compounds
Carbonyl compounds typically display a strong absorption band in the infrared (IR) spectrum, known as the carbonyl stretching vibration. This absorption occurs around 1700 cm^-1 and is a characteristic fingerprint for the presence of a carbonyl group. The exact position of this absorption band can vary slightly depending on the specific functional group within the carbonyl compound (e.g., aldehyde, ketone, ester, carboxylic acid).
In nuclear magnetic resonance (NMR) spectroscopy, the carbon atom directly attached to the carbonyl group in carbonyl compounds often appears at a distinct chemical shift, usually between 190-220 ppm, depending on the compound’s nature and environment. This carbon is referred to as the “carbonyl carbon” and is often used as a diagnostic peak in NMR spectra.
Ketones
Ketones also exhibit a characteristic carbonyl stretching vibration in the IR spectrum, usually around 1700 cm^-1, similar to other carbonyl compounds. In NMR spectroscopy, the carbonyl carbon in ketones falls within the same chemical shift range (190-220 ppm) as in other carbonyl compounds.
Spectroscopic techniques, therefore, provide valuable tools for distinguishing carbonyl compounds, including ketones, from other types of organic compounds. The specific spectral patterns and shifts aid in their precise identification and structural elucidation.
6. Isomerism in Carbonyl Compounds and Ketones
Carbonyl Compounds
Carbonyl compounds can exhibit structural isomerism, where the arrangement of atoms within the molecule is different. The most common example of structural isomerism among carbonyl compounds involves the position of the carbonyl group within the molecule. Aldehydes and ketones are constitutional isomers, as they have the same molecular formula (CnH2nO) but different structural arrangements. For instance, formaldehyde (H-CHO) and acetone (CH3-C(=O)-CH3) are constitutional isomers.
Additionally, some carbonyl compounds exhibit tautomeric isomerism. Tautomers are isomers that can interconvert through the migration of a hydrogen atom and a double bond. For example, keto-enol tautomerism is observed in compounds like 2,4-pentanedione, where the keto form and enol form can coexist in equilibrium.
Ketones
Ketones themselves can also exhibit structural isomerism when the alkyl or aryl groups attached to the carbonyl carbon differ. For example, methyl ethyl ketone (CH3-C(=O)-C2H5) and diethyl ketone (CH3-C(=O)-C2H5) are structural isomers of each other.
In summary, both carbonyl compounds and ketones can display various forms of isomerism, adding an extra layer of complexity to their structural diversity and reactivity.
7. Biological Significance
Carbonyl Compounds
Carbonyl compounds are found in a wide range of biomolecules. For example:
- Aldehydes are involved in metabolic pathways such as glycolysis and fatty acid synthesis. Acetaldehyde is a product of ethanol metabolism in the human body.
- Ketones like acetoacetate, beta-hydroxybutyrate, and acetone are produced during the metabolism of fats (lipids) and are important energy sources during periods of fasting or low carbohydrate intake. This metabolic state is known as ketosis.
- Carboxylic acids are abundant in biological systems. They are integral components of amino acids, fatty acids, and many other biomolecules. Citric acid, a carboxylic acid, is a key intermediate in the citric acid cycle, a central metabolic pathway.
Ketones
Ketones, as a subgroup of carbonyl compounds, have specific biological roles:
- Ketosis: Ketones are produced in the liver during periods of fasting or when carbohydrate intake is low. They serve as an alternative energy source for the brain and other tissues when glucose availability is limited. This metabolic state is crucial for survival during extended periods without food.
- Ketone Bodies: The three main ketone bodies produced in the body are acetoacetate, beta-hydroxybutyrate, and acetone. These molecules are transported to various tissues, including the brain, where they are used for energy production.
- Ketone Testing: The presence of ketone bodies in urine or blood can indicate various health conditions, including uncontrolled diabetes or ketogenic diets. Ketone testing is a valuable diagnostic tool in clinical settings.
8. Safety Considerations
Carbonyl Compounds
Carbonyl compounds, particularly aldehydes, can exhibit some hazardous properties:
- Flammability: Many aldehydes are highly flammable and can form explosive peroxides upon exposure to air. Proper storage and handling are essential to minimize these risks.
- Irritation: Some aldehydes, such as formaldehyde, can be irritating to the eyes, skin, and respiratory tract. Adequate ventilation and personal protective equipment (PPE) are necessary when working with them.
Ketones
Ketones also come with specific safety considerations:
- Flammability: Ketones like acetone are highly flammable and should be stored away from open flames or heat sources. Proper fire safety protocols should be followed.
- Volatility: Ketones can be volatile and may release vapors that can be harmful if inhaled in high concentrations. Adequate ventilation is crucial when working with volatile ketones.
- Skin Irritation: Some ketones can irritate the skin and eyes. The use of appropriate PPE, including gloves and safety goggles, is recommended.
It’s essential to be aware of these safety considerations and follow established safety protocols when handling carbonyl compounds and ketones in laboratory and industrial settings.
9. Environmental Impact
Carbonyl Compounds
- Formaldehyde, an aldehyde, is a volatile organic compound (VOC) and can contribute to air pollution. It is regulated due to its potential health and environmental risks.
- Acetone, a ketone, is relatively less harmful to the environment but can contribute to air pollution if released in significant quantities.
Ketones
- Environmental Impact: Ketones, as a group, have a relatively low environmental impact compared to some other chemical classes. However, the environmental effects can vary depending on the specific ketone and its use.
- Solvent Use: Ketones like acetone are commonly used as solvents in various industries. Proper disposal and recycling practices are essential to minimize environmental contamination.
In recent years, there has been a growing emphasis on developing greener and more sustainable chemical processes, which includes reducing the environmental impact of chemicals like carbonyl compounds and ketones.
10. Synthesis and Applications
Carbonyl Compounds
Carbonyl compounds are essential in various synthesis routes and applications:
- Pharmaceuticals: Carbonyl compounds serve as key intermediates in the synthesis of pharmaceuticals. The ability to modify and functionalize carbonyl groups is crucial in drug development.
- Polymers: They are used in the production of polymers and plastics. For example, formaldehyde is a vital building block for phenolic resins used in plastics and adhesives.
- Flavors and Fragrances: Aldehydes and ketones contribute to the scents and flavors of numerous natural and synthetic products. They play a significant role in the perfume and food industries.
Ketones
Ketones have specific applications:
- Solvents: Ketones such as acetone and methyl ethyl ketone (MEK) are commonly used as industrial solvents. Acetone, in particular, is a versatile solvent utilized in various applications.
- Pharmaceuticals: Ketones are crucial in pharmaceutical synthesis. They are used as intermediates in the production of pharmaceutical compounds.
- Ketogenic Diet: Ketones are a primary source of energy during a ketogenic diet, which is used for weight loss and managing certain medical conditions. The diet induces a state of ketosis, where the body relies on ketone bodies for energy.
11. Carbonyl Compounds and Ketones in Nature
Carbonyl Compounds
- Photosynthesis: Carbonyl compounds like glucose and fructose are crucial products of photosynthesis in plants. They serve as energy storage molecules.
- Metabolism: Aldehydes are intermediate products in various metabolic pathways, including glycolysis. Acetaldehyde is a product of ethanol metabolism in the human body.
- Scent and Flavor: Some aldehydes, like benzaldehyde, contribute to the characteristic scents and flavors of fruits and nuts.
Ketones
- Ketosis: Ketones are naturally produced by the liver during fasting or low carbohydrate intake. This metabolic state, known as ketosis, is crucial for providing energy to the body and brain when glucose is limited.
- Energy Source in Animals: Ketones are essential energy sources for animals during periods of fasting or hibernation. They help spare glucose for critical tissues like the brain.
- Ketone Bodies: Acetoacetate, beta-hydroxybutyrate, and acetone are the primary ketone bodies produced in the body. They play essential roles in energy metabolism.
12. Environmental Impact and Sustainability
Carbonyl Compounds
- Formaldehyde, an aldehyde, is a volatile organic compound (VOC) that can contribute to air pollution. It is subject to environmental regulations due to potential health and environmental risks.
- Acetone, a ketone, is less harmful to the environment but can contribute to air pollution when released in significant quantities.
Ketones
- Environmental Impact: Ketones, as a group, have a relatively low environmental impact compared to some other chemical classes. However, their environmental effects can vary depending on the specific ketone and its use.
- Solvent Use: Ketones like acetone are commonly used as solvents in various industries. Proper disposal and recycling practices are essential to minimize environmental contamination.
13. Safety Considerations
Carbonyl Compounds
- Flammability: Many aldehydes are highly flammable and can form explosive peroxides when exposed to air. Proper storage and handling are essential to minimize these risks.
- Irritation: Some aldehydes, such as formaldehyde, can be irritating to the eyes, skin, and respiratory tract. Adequate ventilation and personal protective equipment (PPE) are necessary when working with them.
Ketones
- Flammability: Ketones like acetone are highly flammable and should be stored away from open flames or heat sources. Proper fire safety protocols should be followed.
- Volatility: Ketones can be volatile and may release vapors that can be harmful if inhaled in high concentrations. Adequate ventilation is crucial when working with volatile ketones.
- Skin Irritation: Some ketones can irritate the skin and eyes. The use of appropriate PPE, including gloves and safety goggles, is recommended.
14. Future Perspectives
The fields of organic chemistry, biochemistry, and chemical engineering continue to advance, and the understanding of carbonyl compounds and ketones is central to these developments. Future research is likely to focus on:
- Green Chemistry: Developing more environmentally friendly synthesis routes and reducing the environmental impact of carbonyl compounds and ketones.
- Drug Development: Further exploration of carbonyl compounds’ roles in drug development and the discovery of new pharmaceuticals.
- Metabolism and Health: Investigating the effects of ketosis on health and exploring potential therapeutic applications.
FAQs
A carbonyl compound is a class of organic molecules containing a carbon atom double-bonded to an oxygen atom (C=O). Ketones are a specific subgroup of carbonyl compounds in which the C(=O) bond is located between two carbon-containing groups. So, while all ketones are carbonyl compounds, not all carbonyl compounds are ketones.
Carbonyl compounds, including ketones, are generally polar due to the electronegativity difference between carbon and oxygen in the C=O bond. This polarity leads to higher boiling and melting points compared to nonpolar compounds of similar size. Ketones typically have lower boiling and melting points than carboxylic acids and aldehydes of similar size.
Both carbonyl compounds and ketones exhibit similar reactivity patterns, including nucleophilic addition reactions. However, ketones are generally more resistant to oxidation compared to other carbonyl compounds. This resistance makes ketones valuable intermediates in organic synthesis.
Carbonyl compounds, including ketones, exhibit a strong absorption band in the infrared (IR) spectrum around 1700 cm^-1 due to the carbonyl stretching vibration. In nuclear magnetic resonance (NMR) spectroscopy, the carbon atom directly attached to the carbonyl group in carbonyl compounds typically appears at a distinct chemical shift, usually between 190-220 ppm, which is often used for identification.
Carbonyl compounds find applications in pharmaceuticals, perfumery, the food industry, and polymer production. Ketones are used as solvents, pharmaceutical intermediates, flavor and fragrance contributors, and essential building blocks in organic synthesis.
Some carbonyl compounds, like formaldehyde, can contribute to air pollution. Ketones have a relatively low environmental impact but should be handled and disposed of properly to minimize contamination.
Yes, safety is crucial. Some carbonyl compounds, particularly aldehydes, can be flammable and irritating. Ketones like acetone are also flammable and volatile, requiring proper precautions, such as adequate ventilation and personal protective equipment.
To delve deeper into the distinctions and nuances between carbonyl compounds and ketones, you can explore comprehensive resources in organic chemistry textbooks, online courses, and research articles.
Read More:
Contents
- Differences Between Carbonyl and Ketone
- 1. The Molecular Identities
- 2. Physical Properties
- 3. Reactivity and Chemical Behavior
- 4. Practical Applications
- 5. Spectroscopic Analysis
- 6. Isomerism in Carbonyl Compounds and Ketones
- 7. Biological Significance
- 8. Safety Considerations
- 9. Environmental Impact
- 10. Synthesis and Applications
- 11. Carbonyl Compounds and Ketones in Nature
- 12. Environmental Impact and Sustainability
- 13. Safety Considerations
- 14. Future Perspectives
- FAQs