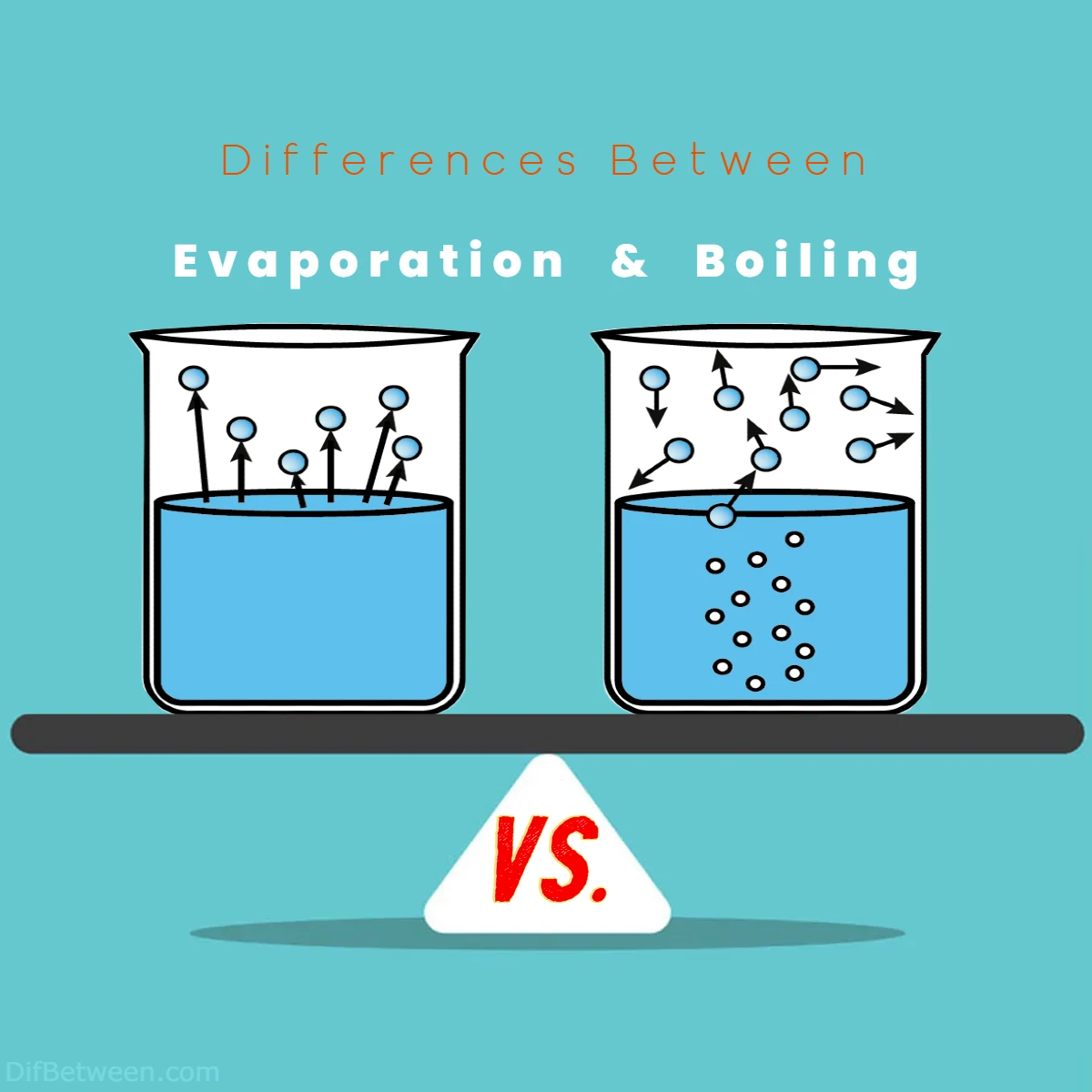
| Characteristic | Evaporation | Boiling |
|---|---|---|
| Temperature | Below the boiling point of the liquid | At the boiling point of the liquid |
| Speed | Slow | Rapid |
| Location | Surface of the liquid | Throughout the entire liquid volume |
| Bubbles | Absent | Present |
| Process | Gradual | Sudden |
| Energy Requirement | Lower energy input | Higher energy input |
| Occurrence in Nature | Continuous | Occurs when the boiling point is reached |
| Environmental Impact | Regulates water cycle, cooling effect | Energy consumption, potential water loss |
| Scientific Advancements | Molecular insights, phase change materials | Heat exchangers, space exploration |
| Future Challenges | Water management, climate modeling | Energy efficiency, advanced materials |
Evaporation and boiling, two processes that share the same liquid-to-gas transformation, couldn’t be more different in their execution. Picture this: on a scorching summer day, you notice a puddle slowly vanishing into thin air, and at dinnertime, the pot of water on your stove is bubbling and hissing as you prepare a hearty meal. From the gentle dance of molecules on a liquid’s surface during evaporation to the dynamic eruption of bubbles in a rolling boil, we’ll traverse the landscapes of temperature, speed, location, and more.
Differences Between Evaporation and Boiling
The main differences between evaporation and boiling lie in their temperature, speed, location, and the presence of bubbles. Evaporation occurs at temperatures below a liquid’s boiling point, happening gradually at the liquid’s surface without bubbles. It’s a slow process driven by ambient heat energy. On the other hand, boiling occurs precisely at the boiling point, throughout the liquid volume, and involves rapid bubble formation, making it more energetic and immediate. These distinctions highlight how these two processes, while both transitioning from liquid to vapor, exhibit distinct characteristics and are fundamental to various aspects of our daily lives and scientific understanding.
Understanding the Basics
Evaporation: A Gentle Transition
Evaporation is a natural process in which a liquid changes into a gas at a temperature below its boiling point. You’ve undoubtedly witnessed this phenomenon countless times—think of a puddle slowly disappearing on a sunny day or the scent of rain-soaked earth after a storm. It’s a ubiquitous occurrence that plays a vital role in our environment.
How Evaporation Works
At the molecular level, evaporation is a bit like a dance. The molecules in a liquid are in constant motion. Some of them possess enough energy to break free from the attractive forces of the other molecules and escape into the air as vapor. This process continues until the liquid is completely transformed into a gas.
Key Characteristics of Evaporation:
- Temperature: Evaporation occurs at a wide range of temperatures, typically below the boiling point of the liquid.
- Speed: It’s a slow process, with molecules escaping the liquid’s surface one by one.
- Location: Evaporation primarily happens at the surface of a liquid.
- Bubbles: Unlike boiling, evaporation doesn’t produce bubbles within the liquid.
Now that we have a solid grasp of evaporation, let’s turn our attention to boiling.
Boiling: A Vigorous Transformation
Boiling, on the other hand, is a much more dramatic process. It occurs when a liquid rapidly changes into vapor, and it usually happens at a specific temperature called the boiling point. Boiling is a more energetic event compared to evaporation and often involves the formation of bubbles.
How Boiling Works
Boiling is all about achieving a balance between the pressure applied to the liquid and the vapor pressure of the liquid itself. When the pressure on the liquid equals or exceeds the vapor pressure, bubbles form within the liquid. These bubbles rise to the surface and release vapor into the surrounding atmosphere. It’s this bubbling action that distinguishes boiling from evaporation.
Key Characteristics of Boiling:
- Temperature: Boiling occurs at a specific temperature known as the boiling point, which is unique to each substance. For water at sea level, this temperature is 100°C (212°F).
- Speed: Boiling is a rapid process, with bubbles forming and rising quickly.
- Location: Boiling happens throughout the entire volume of the liquid.
- Bubbles: Boiling is characterized by the formation of bubbles within the liquid.
Now that we’ve covered the basics, let’s delve deeper into the key differences between evaporation and boiling.
A Side-by-Side Comparison
To better understand the distinctions between evaporation and boiling, let’s summarize their differences in a handy table:
| Characteristic | Evaporation | Boiling |
|---|---|---|
| Temperature | Below the boiling point of the liquid | At the boiling point of the liquid |
| Speed | Slow | Rapid |
| Location | Surface of the liquid | Throughout the entire liquid volume |
| Bubbles | Absent | Present |
| Process | Gradual | Sudden |
| Energy Requirement | Lower energy input | Higher energy input |
| Occurrence in Nature | Continuous | Occurs when the boiling point is reached |
Now that we have a clear overview, let’s explore these differences in more detail.
Temperature: Cool vs. Hot
One of the most fundamental distinctions between evaporation and boiling is the temperature at which they occur.
Evaporation: Below the Boiling Point
Evaporation takes place at temperatures below the boiling point of the liquid. This means that even on a relatively cool day, you can observe evaporation happening. It’s a gradual process driven by the heat energy available in the environment.
Evaporation plays a crucial role in regulating Earth’s climate and the water cycle. For example, it’s responsible for water evaporating from oceans, forming clouds, and eventually falling as rain.
Boiling: At the Boiling Point
Boiling, in contrast, happens at the specific temperature known as the boiling point of the liquid. This temperature varies depending on the substance and the pressure of the surroundings. For instance, water boils at 100°C (212°F) at sea level, but the boiling point changes with altitude.
Boiling is a more energetic event, requiring the liquid to reach a certain temperature threshold before it transitions into vapor. This temperature is consistent and characteristic of the substance, making it a valuable tool in chemistry and cooking.
Speed: Slow and Steady vs. Quick and Dynamic
The speed at which a liquid transforms into vapor is another significant difference between evaporation and boiling.
Evaporation: Slow and Steady
Evaporation is a gradual process. Molecules on the surface of the liquid gain enough energy over time to break free from the liquid’s surface tension and transition into vapor. This happens one molecule at a time, making evaporation relatively slow compared to boiling.
Think of a wet towel hung out to dry or a puddle slowly disappearing on a sunny day—these are everyday examples of evaporation in action. The slow pace of evaporation allows it to occur at lower temperatures, making it an essential part of Earth’s natural processes.
Boiling: Quick and Dynamic
Boiling, on the other hand, is a rapid and dynamic process. Once the liquid reaches its boiling point, bubbles of vapor form within the liquid and rise to the surface. As these bubbles burst at the surface, they release vapor into the surrounding environment.
Boiling is an unmistakable event due to its vigorous bubbling and the rapid conversion of the liquid into vapor. It’s this quick transformation that sets boiling apart from the more gradual process of evaporation.
Location: Surface vs. Entire Volume
Where these transformations occur within the liquid is another crucial distinction between evaporation and boiling.
Evaporation: Surface Phenomenon
Evaporation primarily takes place at the surface of the liquid. It’s the molecules at the liquid’s surface that gain enough energy to break free and become vapor. As these molecules escape, they leave behind slightly cooler and denser liquid molecules, which is why evaporation has a cooling effect.
You can observe this surface phenomenon when a liquid, like water, is left exposed to the air. Over time, the liquid level decreases as molecules continuously evaporate from the top layer.
Boiling: Throughout the Liquid
Boiling, in contrast, occurs throughout the entire volume of the liquid. When the liquid reaches its boiling point, bubbles form at various depths within the liquid, not just at the surface. These bubbles rise through the liquid, releasing vapor as they ascend, and eventually reach the surface, where they burst.
The fact that boiling is not limited to the surface is one reason it’s considered a more energetic and rapid process than evaporation.
Bubbles: Absent vs. Present
The presence or absence of bubbles is a clear visual indicator that distinguishes evaporation from boiling.
Evaporation: Bubbles Are Absent
Evaporation does not involve the formation of bubbles within the liquid. Instead, it’s a relatively quiet and inconspicuous process where individual molecules gain enough energy to transition from liquid to vapor without causing noticeable agitation in the liquid.
Next time you leave a glass of water on the kitchen counter, notice that it doesn’t bubble like a pot of boiling water on the stove. Instead, it gradually disappears as molecules evaporate from the surface.
Boiling: Bubbles Are Present
Boiling, on the other hand, is characterized by the formation of bubbles within the liquid. As the liquid reaches its boiling point, the pressure within the liquid equals or exceeds the vapor pressure, leading to the rapid formation of vapor-filled bubbles. These bubbles rise to the surface and release vapor into the atmosphere.
Whether you’re boiling water for pasta or heating a pot of soup, the presence of bubbles is a sure sign that you’ve reached the boiling point.
Process: Gradual vs. Sudden
Another way to differentiate between evaporation and boiling is by looking at the nature of the processes themselves.
Evaporation: Gradual Transition
Evaporation is a gradual transition from liquid to vapor. It’s a process that occurs slowly over time as individual molecules gain sufficient energy to escape from the liquid’s surface. This gradual nature is why evaporation is often described as a “gentle” transformation.
Because of its gradual pace, evaporation is a practical means of cooling, whether it’s the natural cooling of sweat on your skin or the cooling effect of a fan blowing over a wet surface.
Boiling: Sudden Transformation
Boiling, in contrast, is a sudden and more dramatic transformation. It’s an event that occurs rapidly once the liquid reaches its boiling point. This abrupt shift from liquid to vapor is marked by the formation of bubbles and the release of vapor into the surrounding environment.
Boiling is commonly used in cooking to prepare various dishes, and it’s a crucial part of processes like pasteurization and sterilization, where quick and complete transformation is essential.
Energy Requirement: Lower vs. Higher Input
The energy required for evaporation and boiling varies, with boiling demanding more energy due to its rapid nature.
Evaporation: Lower Energy Input
Evaporation requires a relatively lower energy input compared to boiling. Since it’s a slow and gradual process, the molecules at the surface only need to acquire a modest amount of energy to transition into vapor. This makes evaporation accessible even at lower temperatures.
The energy for evaporation typically comes from the surrounding environment, such as sunlight warming a puddle or the heat from your body causing sweat to evaporate.
Boiling: Higher Energy Input
Boiling, on the other hand, demands a significantly higher energy input. To reach the boiling point and maintain it, you need to supply sufficient heat to the entire volume of the liquid. This heat energy must be continually applied to keep the liquid at or above its boiling point.
In a kitchen setting, this energy is usually supplied by a stove or other heating element. The intensity of the heat source directly affects the rate of boiling.
Occurrence in Nature: Continuous vs. Temperature-Dependent
The occurrence of evaporation and boiling in nature is also worth noting.
Evaporation: Continuous Process
Evaporation is a continuous process that can happen at any temperature below the boiling point of a liquid. It occurs naturally in various environmental conditions, contributing to the water cycle and maintaining the balance of moisture in the atmosphere.
Whether it’s a dewy morning or a sunny afternoon, evaporation is at work, ensuring that our planet’s water resources are constantly on the move.
Boiling: Occurs at the Boiling Point
Boiling, in contrast, occurs only when the liquid reaches its specific boiling point. This means it is temperature-dependent and doesn’t happen continuously at lower temperatures. Boiling is often a deliberate action, such as heating water to cook pasta or pasteurizing milk.
While evaporation is an ongoing natural process, boiling is a controlled process primarily initiated by humans for various purposes.
Factors Influencing Evaporation and Boiling
Now that we’ve thoroughly explored the differences between evaporation and boiling, it’s important to consider the factors that can influence these processes.
Factors Affecting Evaporation
- Temperature: Higher temperatures increase the rate of evaporation. The greater the thermal energy in the environment, the more molecules in the liquid gain enough energy to transition into vapor.
- Surface Area: Increasing the exposed surface area of the liquid accelerates evaporation. This is why a wet cloth spread out dries faster than a puddle in a confined space.
- Humidity: Lower humidity levels in the surrounding air encourage faster evaporation. Dry air can absorb more moisture, facilitating the process.
- Air Movement: Wind or air circulation promotes evaporation by continually removing the vapor-saturated air from the liquid’s surface.
- Pressure: Changes in atmospheric pressure can affect the rate of evaporation. Lower pressure environments, such as those at higher altitudes, can lead to faster evaporation.
Factors Affecting Boiling
- Temperature: Boiling occurs when the liquid reaches its boiling point, so increasing the temperature of the liquid will expedite boiling.
- Pressure: Changes in pressure can alter the boiling point of a substance. For example, water boils at a lower temperature at higher altitudes due to decreased atmospheric pressure.
- Heating Source: The intensity of the heating source affects how quickly a liquid reaches its boiling point. Higher heat settings on a stove or a more powerful heating element speed up the boiling process.
- Container: The material and shape of the container can influence boiling. Some materials conduct heat more efficiently, leading to faster boiling.
- Agitation: Stirring or agitating the liquid can promote boiling by distributing heat more evenly throughout the liquid.
Practical Applications
Understanding the differences between evaporation and boiling is not just a matter of scientific curiosity; it has practical applications in various aspects of our lives.
Everyday Uses of Evaporation
- Cooling Mechanism: Evaporation is the underlying principle behind cooling mechanisms like sweat. When you sweat, moisture on your skin evaporates, taking away heat and providing a cooling effect.
- Drying Clothes: Hanging wet clothes in the sun or using a dryer relies on evaporation to remove moisture and dry the fabric.
- Food Preservation: Food preservation methods like drying and dehydration harness the power of evaporation to extend the shelf life of fruits, vegetables, and meat.
Everyday Uses of Boiling
- Cooking: Boiling is a fundamental cooking technique used to prepare a wide range of dishes, from pasta and rice to soups and stews.
- Sterilization: In medical and laboratory settings, boiling is employed to sterilize equipment and materials, ensuring they are free from harmful microorganisms.
- Water Purification: Boiling water is an effective method of purifying it, as the high temperatures kill bacteria, viruses, and parasites.
- Chemical Reactions: In chemistry, boiling is used to carry out various reactions that require precise temperature control.
Environmental Impact
Beyond their everyday applications, both evaporation and boiling play essential roles in environmental processes.
Environmental Impact of Evaporation
- Water Cycle: Evaporation is a cornerstone of the Earth’s water cycle. It’s responsible for the transfer of water from the Earth’s surface to the atmosphere, where it eventually condenses to form clouds and falls as precipitation.
- Climate Regulation: Evaporation helps regulate the Earth’s temperature. When water evaporates, it absorbs heat energy from the surroundings, cooling the environment. This cooling effect is particularly significant in bodies of water, where evaporation helps maintain stable temperatures.
- Ecosystems: Many ecosystems, such as wetlands and mangroves, depend on evaporation to maintain water levels and provide habitats for various species.
Environmental Impact of Boiling
- Water Conservation: Boiling water for purification or cooking is a common practice, but it often leads to water loss through steam. While boiling can kill harmful microorganisms, alternative water treatment methods that conserve water, such as filtration and chemical disinfection, are more sustainable choices.
- Energy Consumption: Boiling requires a significant amount of energy, especially when preparing food on a large scale or in industries. Energy-efficient cooking methods, such as steaming or using pressure cookers, can help reduce the environmental impact.
- Waste Generation: In industrial processes, boiling may generate waste byproducts or emissions, depending on the substances being boiled. Proper waste management and emission control are crucial to mitigate these environmental effects.
Scientific and Technological Advancements
Scientific research and technological advancements have expanded our understanding of evaporation and boiling, leading to innovations and applications in various fields.
Scientific Advancements
- Molecular Insights: Advances in molecular dynamics simulations and experimental techniques have provided deeper insights into the behavior of molecules during evaporation and boiling, allowing scientists to better understand these processes at the atomic level.
- Phase Change Materials: Researchers have developed phase change materials (PCMs) based on the principles of evaporation and condensation. These materials are used in thermal energy storage systems, helping to improve energy efficiency in heating and cooling applications.
Technological Applications
- Heat Exchangers: Evaporation and boiling principles are employed in heat exchangers, which are crucial components in refrigeration and air conditioning systems. Modern heat exchangers are designed for enhanced efficiency and reduced environmental impact.
- Boiling in Microgravity: Understanding boiling in microgravity environments has applications in space exploration. Researchers study how boiling behaves differently in space, leading to advancements in spacecraft cooling systems and life support technology.
- Nanotechnology: Nanotechnology has opened up new possibilities for controlling and enhancing heat transfer during evaporation and boiling processes. This has implications for more efficient cooling systems in electronics and advanced materials development.
Future Challenges and Research Areas
As our world faces increasing challenges related to energy efficiency, sustainability, and climate change, research in evaporation and boiling continues to be relevant. Here are some future challenges and research areas in these fields:
Evaporation Research
- Water Management: With growing concerns about water scarcity, research into improving the efficiency of desalination and wastewater treatment through evaporation-driven processes is critical.
- Climate Modeling: Understanding the role of evaporation in climate regulation is essential for accurate climate modeling and predictions.
- Renewable Energy: Exploring innovative ways to harness the energy generated by evaporation-driven processes, such as evaporative cooling and solar desalination, can contribute to sustainable energy solutions.
Boiling Research
- Energy Efficiency: Developing more energy-efficient boiling techniques for industrial applications can reduce energy consumption and greenhouse gas emissions.
- Nuclear Safety: Research into the behavior of cooling systems involving boiling in nuclear reactors is crucial for enhancing safety and preventing accidents.
- Advanced Materials: Investigating new materials that promote boiling with minimal energy input can lead to improved heat exchangers and thermal management systems.
In conclusion, evaporation and boiling are fundamental processes with distinct characteristics, applications, and environmental impacts. Understanding these processes is not only essential for scientific knowledge but also for addressing real-world challenges related to energy efficiency, environmental sustainability, and water management. As research in these fields continues to advance, we can expect new innovations and solutions to emerge, contributing to a more sustainable and efficient future.
FAQs
The primary difference is the temperature at which they occur. Evaporation happens below the boiling point of a liquid, while boiling occurs precisely at the boiling point.
Evaporation is slow and gradual, with molecules escaping from the liquid’s surface one by one. Boiling is rapid, characterized by the formation of bubbles and quick conversion of the liquid into vapor.
Evaporation primarily occurs at the surface of the liquid, while boiling happens throughout the entire volume of the liquid.
Bubbles are absent during evaporation, but they are a defining feature of boiling, where they form within the liquid.
Evaporation requires lower energy input as it’s a gradual process. Boiling demands higher energy input due to its rapid and energetic nature.
Yes, both processes have environmental impacts. Evaporation plays a role in regulating the water cycle and climate, while boiling can lead to energy consumption and potential water loss.
Evaporation research explores water management and climate modeling, while boiling research focuses on energy efficiency and advanced materials. Both fields offer avenues for innovation and sustainability.
Read More:
Contents
- Differences Between Evaporation and Boiling
- Understanding the Basics
- A Side-by-Side Comparison
- Temperature: Cool vs. Hot
- Speed: Slow and Steady vs. Quick and Dynamic
- Location: Surface vs. Entire Volume
- Bubbles: Absent vs. Present
- Process: Gradual vs. Sudden
- Energy Requirement: Lower vs. Higher Input
- Occurrence in Nature: Continuous vs. Temperature-Dependent
- Factors Influencing Evaporation and Boiling
- Practical Applications
- Environmental Impact
- Scientific and Technological Advancements
- Future Challenges and Research Areas
- FAQs



