Analytical Chemistry
Welcome to our comprehensive collection of content dedicated to the fascinating realm of Analytical Chemistry! If you’re curious about the intricacies and nuances of this scientific field, you’ve come to the right place. In this category, we delve deep into the realm of Analytical Chemistry, highlighting the differences in terms, methodologies, and other essential elements that make this discipline both captivating and indispensable.
Analytical Chemistry plays a vital role in understanding the composition, properties, and behavior of matter. It involves the application of various techniques and tools to identify and quantify chemical compounds, elucidate their structures, and uncover their roles in diverse fields, ranging from environmental studies to pharmaceutical development.
In this exploration of Analytical Chemistry, we’ll traverse a wide range of topics, unraveling the differences in terms and other items that are central to this field. Whether you’re a student, researcher, or simply an inquisitive mind, our carefully curated content will provide you with valuable insights and deepen your understanding of Analytical Chemistry.
Now, let’s embark on our journey through the captivating world of Analytical Chemistry, where we’ll encounter a multitude of concepts, techniques, and terminologies that are fundamental to the discipline.
-

Molarity vs Concentration
In the intricate world of chemistry, two fundamental concepts often steal the spotlight: Concentration and Molarity. They might appear synonymous at first glance, both pertaining to the measure of solute in a solution, but beneath the surface, they diverge into distinct entities. Let's embark on a journey to dissect and comprehend the intriguing Differences Between Concentration vs Molarity. Concentration, a versatile term, encapsulates various expressions to quantify the solute's presence within a solution. Whether it's mass percentage, volume percentage, or mole fraction, concentration provides insights into the solution's "strength" or "intensity." This concept plays a pivotal role in diverse applications, from concocting culinary delights in the kitchen to monitoring environmental pollutants in the great outdoors. Molarity (M), on the other hand, hones in on precision. It's all about the number of moles of solute per liter of solution. When you need exactitude in chemical reactions, especially in stoichiometry, molarity becomes the go-to unit. It facilitates precise control of reactant quantities, ensuring that chemical equations unfold as planned.
-

Titre vs Dilution
In the realm of laboratory science, precision and accuracy reign supreme, and two fundamental techniques, dilution and titration, stand as pillars of analytical prowess. These methods, though related to the handling of solutions, serve vastly different purposes and involve distinct processes. Dilution, like a gentle brushstroke on a canvas, is the art of reducing the concentration of a solution by introducing a solvent. It is used to tailor solutions for various reasons, including safety, precision, and cost-efficiency. The process relies on mathematical calculations, making it a straightforward and essential technique in fields such as pharmaceuticals, chemical analysis, and biological research. In contrast, titration is akin to a scientific detective's quest for answers. It is a meticulously controlled chemical reaction between an analyte of unknown concentration and a titrant of known concentration. The key to titration lies in the precise detection of the endpoint, often signaled by a dramatic change in color due to the use of indicators. This technique is paramount in analytical chemistry, enabling scientists to unravel the mysteries of unknown concentrations and ensure product quality control in the pharmaceutical industry.
-

Fast HPLC vs HPLC
When it comes to liquid chromatography, two powerful techniques stand out: High-Performance Liquid Chromatography (HPLC) and its rapid sibling, Fast HPLC (High-Speed or Ultra-Fast Liquid Chromatography). These analytical methods share a common goal—separating and quantifying compounds within complex mixtures—but they diverge significantly in their approach. HPLC, characterized by its meticulous precision and methodical pace, excels in delivering high-resolution separations. It's the choice for applications where every detail matters, from pharmaceutical quality control to in-depth research involving intricate sample matrices. In contrast, Fast HPLC, as its name suggests, prioritizes speed without sacrificing resolution to the same degree. This technique is the go-to option for high-throughput laboratories where efficiency and rapid results are paramount. Delving into the nuances reveals that HPLC operates at moderate pressures, typically around 6,000 to 7,000 psi, while Fast HPLC boldly ventures into the high-pressure realm, often exceeding 10,000 psi. This higher pressure environment in Fast HPLC is the trade-off for its remarkable speed, demanding robust instrumentation capable of handling the stress. Sample size handling also differs, with HPLC offering versatility across a wide range of volumes and Fast HPLC optimizing for smaller to medium-sized sample volumes.
-

GC vs HPLC
In the realm of analytical chemistry, the choice between High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC) can be as critical as choosing the right tool for a complex task. Both HPLC and GC are indispensable techniques used to separate, identify, and quantify compounds in a variety of samples, but they employ different principles and are suited for distinct applications. HPLC, as the name suggests, employs a liquid mobile phase, making it highly versatile. It can handle a wide range of sample types, from liquids to solids, and is renowned for its compatibility with non-volatile compounds. This makes HPLC a valuable tool in industries like pharmaceuticals, food and beverage, clinical diagnostics, and environmental monitoring. While it offers moderate sensitivity, HPLC's ability to handle diverse samples and its robustness make it a popular choice for routine analytical tasks. On the other side of the spectrum, we have GC, a technique that relies on a vaporized gas mobile phase. This characteristic makes GC exceptionally well-suited for the analysis of volatile compounds. It's celebrated for its high sensitivity, capable of detecting trace-level compounds with precision. GC finds its niche in applications such as environmental monitoring, where minute concentrations of pollutants need to be identified, and in forensic science, where the detection of volatile substances plays a crucial role.
-

UPLC vs HPLC
When it comes to chromatography, precision is paramount. High-Performance Liquid Chromatography (HPLC) and Ultra-Performance Liquid Chromatography (UPLC) are two prominent techniques in the world of analytical chemistry. In our detailed analysis, we uncover the primary distinctions between these chromatographic methods. From particle sizes and operating pressures to resolution, sensitivity, and cost considerations, we break down the factors that can influence your choice between HPLC and UPLC. HPLC, with its versatile and cost-effective approach, remains a steadfast choice for various applications. On the other hand, UPLC's cutting-edge technology offers exceptional speed, resolution, and sensitivity, making it the preferred option for demanding analyses. Whether you prioritize reliability, versatility, and affordability or require blazing speed and precision, our exploration of HPLC vs UPLC will guide you toward the chromatographic technique best suited to your laboratory's objectives.
-

Bromocresol Purple vs Bromocresol Blue
In the realm of chemistry and laboratory experiments, nuances matter. Enter Bromocresol Blue and Bromocresol Purple, two pH indicators that might look similar but play very different roles. Bromocresol Blue, with its vibrant blue to yellow shift, thrives in the pH range of 6.0 to 7.6, making it a go-to for detecting slight pH changes. On the other hand, Bromocresol Purple, transitioning from yellow to purple in the pH range of 5.2 to 6.8, is your choice for pinpoint accuracy in mildly acidic to mildly alkaline solutions. Dive into the colorful world of these indicators, understand their unique properties, and choose the right one for your experiments. Whether you're a seasoned scientist or an aspiring chemist, this comparison will help you make informed decisions and uncover the secrets of pH monitoring in the laboratory.
-

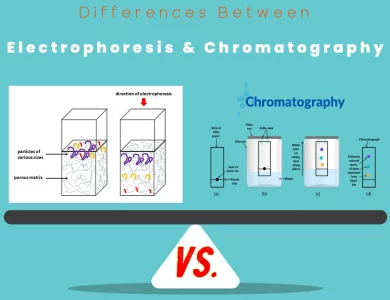
Chromatography vs Electrophoresis
In the realm of analytical chemistry, the choice between electrophoresis and chromatography can be akin to selecting the right tool from a scientist's treasure chest. These two techniques stand as pillars in the world of molecular analysis, each offering a unique set of capabilities that cater to diverse scientific needs. Electrophoresis, often associated with the graceful migration of DNA bands on a gel, operates on the fundamental principle of charged particle movement in an electric field. This technique is a powerhouse for the separation of charged molecules, including DNA, RNA, proteins, and peptides. It excels at resolving biomolecules based on their size, shape, and charge, making it indispensable in molecular biology, biochemistry, and forensic science. On the other hand, chromatography is like a chameleon in the laboratory, adapting to an extensive range of analytes, both charged and uncharged. Whether you're working with small organic compounds, large biomolecules, or gases, chromatography has you covered. Its magic lies in the differential distribution of compounds between a stationary phase and a mobile phase, guided by properties such as polarity, size, and affinity. This versatility makes chromatography a cornerstone in fields spanning pharmaceuticals, environmental analysis, food science, and beyond.
-

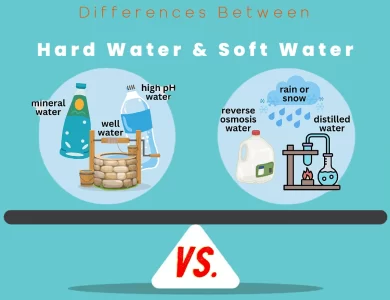
Soft Water vs Hard Water
Water is an essential element in our daily lives, but it comes in two distinct flavors: hard water and soft water. Understanding the differences between them can significantly impact your household, personal well-being, and even your environmental footprint. Hard Water: This type of water is characterized by its high mineral content, primarily calcium and magnesium ions. These minerals find their way into the water as it percolates through rock and soil layers, picking up various substances along the way. The hardness of water is typically measured in parts per million (ppm) or grains per gallon (gpg) of calcium carbonate (CaCO3) equivalents. The consequences of hard water are many. It can lead to scale buildup in plumbing fixtures and appliances, making them less efficient and potentially causing maintenance headaches. Hard water can also affect your skin and hair, leaving them dry and dull. Additionally, it may influence the taste of your food and beverages, as well as the ease of lathering with soap. Soft Water: In contrast, soft water contains fewer dissolved minerals, primarily calcium and magnesium ions. Soft water is often naturally occurring in areas with specific geological formations or can be produced through water softening methods. It's prized for its ability to lather easily with soap and detergents, leaving no scale or residue behind. Soft water is also kinder to your skin and hair, resulting in a moisturizing effect and vibrant hair.
-

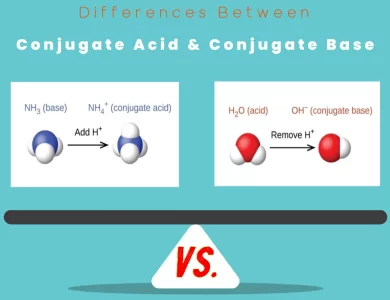
Conjugate Base vs Conjugate Acid
Acid-base chemistry is the backbone of countless chemical reactions, from the laboratory to the natural world. At its heart lie two critical players: conjugate acids and conjugate bases. Understanding their differences is akin to unlocking the secrets of chemical reactivity and the behaviors of substances in diverse environments. Conjugate Acid: Think of this as the "donor" in a chemical tango. Conjugate acids are formed when a base accepts a proton (H+). This proton donation transforms the base into its conjugate acid, changing its chemical character and often rendering it positively charged. In practical terms, conjugate acids increase the acidity of a solution and play pivotal roles in defining the pH levels of substances. Conjugate Base: On the flip side, conjugate bases are like the gracious "receivers" in the chemical dance. They form when an acid donates a proton (H+), resulting in the base's transformation into its conjugate base, typically carrying a negative charge. Conjugate bases tend to elevate the basicity of a solution and are essential players in maintaining chemical equilibrium.
-

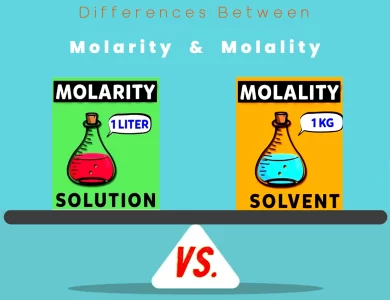
Molality vs Molarity
In the captivating realm of chemistry, two terms often steal the limelight: Molarity and Molality. These two fundamental concepts are pivotal for understanding the concentration of solutions, yet they diverge in significant ways. Molarity, represented as 'M,' quantifies the number of moles of solute per liter of solution and is widely employed in various industries. However, it's worth noting that Molarity is temperature-sensitive due to its reliance on the volume of the solution. On the other hand, Molality, symbolized as 'm,' measures the moles of solute per kilogram of solvent. What sets it apart is its temperature-independence. Molality is favored in scenarios where temperature fluctuations play a critical role, such as in cryogenics or specific chemical reactions. This exploration into the differences between Molarity and Molality will equip you with the knowledge to make informed decisions in the lab or industry, ensuring precise concentration control in your scientific endeavors.
- 1
- 2