Inorganic Chemistry
Welcome to our comprehensive category page dedicated to the intriguing realm of inorganic chemistry! Here, we dive deep into the differences in terms and other fascinating aspects of this captivating branch of science. Whether you’re a curious learner, a student studying chemistry, or an enthusiastic researcher, this is your go-to resource for unraveling the intricacies of inorganic chemistry.
Inorganic chemistry, a subfield of chemistry, focuses on the study of inorganic compounds that do not contain carbon-hydrogen (C-H) bonds. Instead, it delves into the vast array of compounds comprising metals, nonmetals, and their interactions. From the exploration of elements and periodic trends to the understanding of chemical reactions and their applications, inorganic chemistry offers a wealth of knowledge waiting to be explored.
-

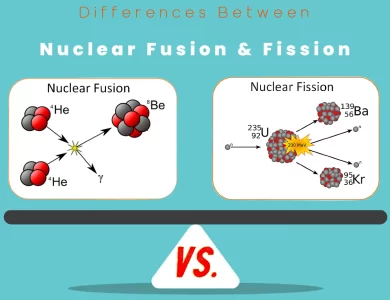
Fission vs Nuclear Fusion
Nuclear energy, with its vast potential and diverse applications, is a topic of immense significance in the quest for clean and sustainable power sources. Two distinct nuclear processes, fusion, and fission, stand at the forefront of this scientific exploration. Understanding the fundamental disparities between these processes is pivotal in unraveling their unique attributes and contributions to the energy landscape. Nuclear fusion, often hailed as the "holy grail" of clean energy, involves the fusion of light atomic nuclei, typically hydrogen isotopes, to form a heavier nucleus. This fusion process mirrors the energy generation mechanism of stars, releasing an extraordinary amount of energy. In contrast, nuclear fission revolves around the splitting of heavy atomic nuclei, like uranium or plutonium, into smaller nuclei, accompanied by the release of substantial energy. Both processes are awe-inspiring in their own right, but they diverge significantly in terms of fuel, energy release, waste generation, and safety. Fusion is characterized by its minimal waste production, low radioactivity, and an almost boundless fuel supply derived from hydrogen isotopes, making it an attractive prospect for a sustainable energy future. Moreover, fusion carries no risk of a nuclear meltdown, enhancing its safety profile. However, it demands the creation and maintenance of extreme conditions, including temperatures in the millions of degrees, which remain a formidable technical challenge. In contrast, fission reactors, which are already operational worldwide, provide a substantial portion of our current energy needs. Yet, they generate significant amounts of radioactive waste, pose safety concerns, and rely on limited fissile materials.
-

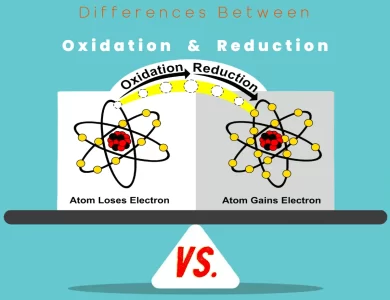
Reduction vs Oxidation
In the captivating realm of chemistry, two fundamental processes, oxidation and reduction, play a pivotal role in shaping the way substances transform and react. Understanding these processes is like unlocking the secrets of chemical reactions, from the rusting of iron to the energy production within our cells. Oxidation is the process where an atom, ion, or molecule loses electrons, leading to an increase in its oxidation state. It's often associated with the addition of oxygen or the removal of hydrogen atoms. Think of it as a substance's willingness to "donate" electrons to other participants in a chemical reaction. On the flip side, reduction is the process where an atom, ion, or molecule gains electrons, causing a decrease in its oxidation state. This can involve the addition of hydrogen or the removal of oxygen. Reduction is akin to a substance's ability to "accept" electrons from other participants in a chemical reaction. These two processes are intricately linked in what we call redox (reduction-oxidation) reactions. In a redox reaction, one substance gets oxidized, losing electrons, while another gets reduced, gaining those electrons. This transfer of electrons is the driving force behind countless chemical reactions, both in the lab and in our daily lives. To gain a deeper understanding of the key distinctions between oxidation and reduction, their roles in chemistry, and their impact on our world, read on in our comprehensive guide. Whether you're a chemistry enthusiast or simply curious about the science that surrounds us, this exploration will shed light on the fascinating world of redox reactions.
-

Potassium Iodide vs Iodine
In the realm of chemistry and health, understanding the differences between iodine and potassium iodide is essential. While both compounds contain the crucial element iodine, they serve distinct roles with unique properties and applications. Iodine, symbolized as I on the periodic table, exists as a purple-black diatomic molecule with a relatively low boiling point. It can be found in seaweed and seafood, making it a dietary essential. Iodine is renowned for its applications in medical antiseptics, dietary supplements, chemical reactions, and even photography, where it contributes to the creation of light-sensitive emulsions. On the other hand, potassium iodide, often abbreviated as KI, is a white crystalline salt, primarily produced synthetically. Its critical role lies in radiation protection during emergencies, safeguarding the thyroid gland from harmful radioactive iodine isotopes. It's also used in pharmaceuticals and plays a crucial part in the iodization of table salt to combat iodine deficiency disorders. In this comprehensive guide, we delve into the chemical properties, health benefits, and potential risks associated with iodine and potassium iodide, shedding light on when and why you might encounter these compounds in your everyday life. So, if you're curious about the distinctions that set these two compounds apart, read on to unravel the captivating world of "Differences Between Iodine vs Potassium Iodide."
-

Iodide vs Iodine
Iodine and iodide, though often used interchangeably, are distinct chemical entities with vital roles in various domains. Iodine, represented as I2, is a diatomic molecule known for its distinctive violet-black crystals and a pungent odor. In contrast, iodide exists as an ion, denoted as I-, typically found in the form of white crystalline salts. These two compounds might appear similar, but their differences are far from subtle. Iodine is crucial for thyroid health, playing a pivotal role in the synthesis of thyroid hormones essential for metabolism regulation. It finds applications in disinfection, chemistry, and even nuclear medicine, where radioactive iodine isotopes aid in diagnostics and therapy. On the other hand, iodide, commonly as potassium iodide (KI), is used in pharmaceuticals to treat respiratory conditions and for radiation protection during nuclear emergencies. It's also a valuable component in analytical chemistry, facilitating redox titrations and precipitation reactions. Understanding the disparities between iodine and iodide empowers us to make informed choices, whether in our health journey, laboratory experiments, or even when preparing for potential environmental contingencies. Join us on this illuminating exploration of iodine and iodide, where we unravel their unique properties and applications, paving the way for a deeper understanding of these essential chemical companions.
-

Diesel vs Petrol
In the world of automotive choices, the age-old debate between petrol and diesel continues to rev its engines. It's a decision that affects not only the way your vehicle performs but also your wallet and the environment. So, let's dive into the fascinating realm of fuel types and explore the nuances that set petrol and diesel apart. At the heart of the petrol vs diesel dilemma are fundamental differences in composition and combustion. Petrol, also known as gasoline, is a highly refined liquid fuel primarily composed of hydrocarbons. It's the spark plug that ignites the controlled explosion within the engine's cylinders, propelling your vehicle forward with a smooth and responsive acceleration. Ideal for city driving and quick starts, petrol offers a refined and efficient ride. On the flip side, diesel fuel boasts a higher energy density, providing better fuel efficiency and longer driving ranges. The compression-ignition process in diesel engines relies on heat generated during compression to ignite the fuel. This unique characteristic results in substantial low-end torque, making diesel engines the go-to choice for long-distance travel, towing heavy loads, and venturing off the beaten path. But the differences don't end there. Maintenance costs, environmental impact, and even the characteristic noise of each engine type play pivotal roles in the petrol vs diesel equation. To make an informed decision for your vehicle, stay tuned as we unravel the intricacies that set these two fuel giants apart. Whether you're a city slicker or an off-road adventurer, the right fuel awaits your choice.
-

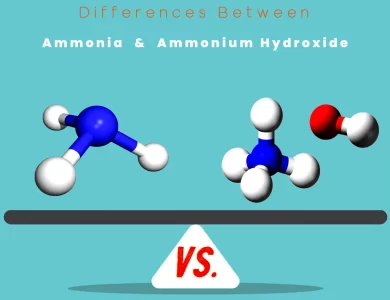
Ammonium Hydroxide vs Ammonia
In the world of chemicals, subtle distinctions can lead to significant differences in properties, applications, and safety considerations. Two such compounds that often cause confusion are ammonia and ammonium hydroxide. While they share a similar pungent odor, they are not the same, and understanding their disparities is crucial, especially when it comes to handling and applications. Ammonia (NH3): This compound is a colorless gas known for its sharp, suffocating smell. It's widely used in various industries, from agriculture as a key component of fertilizers to household cleaning products. Ammonia is also a vital player in industrial refrigeration systems and even finds application in adjusting the pH of solutions. Ammonium Hydroxide (NH4OH): In contrast, ammonium hydroxide is not a standalone compound but rather a liquid solution created by dissolving ammonia gas in water. While it retains that unmistakable ammonia odor, it's in a different physical state. Ammonium hydroxide has diverse applications, from being a cleaning agent in households and industries to serving as a reagent in laboratories. It even plays a role in the food industry for pH adjustment in certain products. Understanding the differences between these two chemicals is not just a matter of semantics but a matter of safety and efficacy in various applications. Whether you encounter them while cleaning your home or in industrial processes, knowing their unique characteristics is essential for making informed decisions and ensuring safe practices.
-

Graphite vs Carbon
Explore the intricate variations between carbon and graphite in this insightful exploration. Carbon, the versatile sixth element, takes on multifaceted forms, from the brilliance of diamonds to the opacity of amorphous substances. On the other hand, graphite showcases a layered elegance, composed of interconnected hexagonal rings that grant it exceptional conductivity and lubricity. These elemental siblings offer distinct properties, bonding types, and even optical characteristics. While carbon displays diverse bonding, graphite's covalent bonds within layers and weak interlayer forces define its conductivity and appearance. In terms of applications, carbon finds its place in a wide range of industries, while graphite excels in roles such as lubricants, electrodes, and pencils. Whether you're intrigued by their mechanical strengths or their thermal conductivities, delving into their differences illuminates the fascinating world of elements and materials science. Join us on a journey to unravel the mysteries that set carbon and graphite apart, shedding light on their diverse roles in shaping our technological landscape.
-

Aluminum vs Iron
Delving into the world of metals, the differences between iron and aluminum come to light, each with its own set of unique properties and applications. Iron, known for its robustness and magnetic allure, finds its strength in construction and machinery. On the other hand, aluminum, with its lightweight yet resilient character, takes center stage in aerospace and modern architecture. Iron's vulnerability to rust contrasts with aluminum's natural corrosion resistance, making it essential to consider the environment when choosing between the two. Industries seeking strength without the heft lean towards iron, while those prioritizing weight reduction turn to aluminum's exceptional strength-to-weight ratio. Recycling prowess is a shared trait, making both metals environmentally sound choices. This journey through the world of iron and aluminum unveils the factors that shape our material choices, reminding us that even in the realm of elements, diversity and innovation abound.
-

Liquid vs Water
Embark on an illuminating journey through the contrasting realms of water and liquid states of matter. While the terms might seem interchangeable, they unveil a tapestry of distinctions that shape our understanding of the building blocks of existence. Water, that life-sustaining elixir, is a specific chemical compound with a molecular formula of H₂O, while "liquid" represents a broader state of matter characterized by its flow and adaptability to its container. Water's remarkable properties, from being a universal solvent to moderating Earth's temperature, set it apart. Meanwhile, the broader concept of liquids encompasses a spectrum of substances, including oils, juices, and more. The interplay between these entities orchestrates the dance of life, from the hydrological cycle to industrial applications. Understanding these differences expands our comprehension of matter's intricacies, bridging the gap between scientific insights and everyday experiences. Join us on this captivating voyage as we delve into the nuanced distinctions between water and liquid, unraveling their roles in nature, culture, and the cosmos beyond. Let's quench our intellectual thirst by delving into the depths of semantics and properties, enriching our perspective on the captivating world of matter in its fluid forms.
-

Zinc vs Zinc Oxide
Embarking on a journey into the world of materials, we delve into the intriguing disparities between zinc and zinc oxide. While they share a common elemental basis, their unique attributes shape their roles in various industries and applications. Zinc, a versatile transition metal, boasts malleability and ductility, making it an ideal candidate for galvanization and alloy production. Its essential micronutrient status in biological systems harmonizes with its potential toxicity when consumed in excess. Environmental considerations highlight the importance of proper disposal to prevent harm to ecosystems. On the other hand, zinc oxide, a compound of zinc and oxygen, dons the form of a white powder with a higher melting point. Its properties, including UV-absorption and mild antiseptic effects, drive its applications in sunscreens, cosmetics, and pharmaceuticals. The safety aspect of zinc oxide is underscored by its Generally Recognized as Safe (GRAS) status, contrasting with zinc's careful dietary moderation. As we venture into their applications, manufacturing methods, and solubility behaviors, a comprehensive understanding of these materials emerges. While zinc finds its place in protective coatings and semiconducting materials, zinc oxide takes center stage in modern technologies, nanoscience, and skincare. In this exploration of zinc vs zinc oxide, we navigate their differences, unveiling the multifaceted nature of materials science and the intricate roles these substances play in shaping our world.
- 1
- 2