General Chemistry
Welcome to our comprehensive category page dedicated to unraveling the intriguing differences in terms and other items in the realm of General Chemistry. Whether you’re a student, a professional in the field, or simply an enthusiast seeking to expand your knowledge, this page is your gateway to understanding the diverse concepts that shape the foundation of this scientific discipline.
Chemistry is a captivating subject that delves into the intricacies of matter and its interactions. It’s a world brimming with fascinating terms, unique elements, and distinctive properties. Through this collection of content, we aim to demystify the often complex jargon, shed light on key differences, and foster a deeper understanding of General Chemistry.
-

Concentration vs Dilution
In the realm of chemistry and everyday life, understanding the nuances between dilution and concentration is paramount. These two processes, seemingly at odds, play vital roles in various fields, from laboratories and healthcare facilities to kitchens and industrial settings. Let's delve into the world of "Differences Between Dilution vs Concentration." Dilution is a process that involves making a solution less concentrated by adding more solvent. The result is a reduction in the amount of solute per unit volume while keeping the total amount of solute constant. Dilution is akin to fine-tuning, allowing precise control over solution strength. It finds application in scientific experiments, where accuracy is paramount, as well as in healthcare, where medications are prepared at specific dosages tailored to individual patients. Dilution also plays a pivotal role in the food and beverage industry, where flavors and sweetness levels are adjusted to meet consumer preferences. Concentration, on the other hand, entails increasing the amount of solute in a solution, often by adding more solute substance or removing some of the solvent. Concentrated solutions boast a higher solute-to-solvent ratio, making them potent and ideal for various applications. Industries like chemical synthesis, food preservation, and pharmaceuticals rely on concentration to achieve desired results, from driving reactions to completion to preserving the shelf life of products.
-

Normal Air vs Nitrogen in Tires
When it comes to tire maintenance, choosing between normal air and nitrogen inflation can impact your vehicle's performance and safety. Understanding the key differences between these two options is crucial for making an informed decision. Composition: Normal air consists of a mix of gases, including 78% nitrogen and 21% oxygen, along with trace elements. In contrast, nitrogen inflation provides nearly pure nitrogen, typically around 95-99% purity. Pressure Stability: Nitrogen-filled tires offer more stable pressure over time. The larger nitrogen molecules are less likely to escape through the tire's rubber, reducing the need for frequent top-ups, especially when compared to the oxygen permeation in normal air. Moisture Content: Normal air contains water vapor, which can lead to moisture accumulation within the tire. Nitrogen, being dry, prevents internal corrosion and rusting of tire components. Temperature Effects: Nitrogen is less sensitive to temperature changes, ensuring consistent pressure regardless of weather conditions. In contrast, normal air experiences significant pressure fluctuations with temperature variations. Permeation Rate: Nitrogen molecules have a slower leak rate compared to oxygen, resulting in more prolonged periods between pressure checks and top-ups. Availability and Cost: Normal air is readily accessible and often free at gas stations. Nitrogen inflation may come at a cost due to specialized equipment and materials, making it less convenient for some. Environmental Impact: Nitrogen is environmentally friendly, lacking harmful emissions and greenhouse gases found in normal air. By weighing these factors, you can decide whether nitrogen or normal air best suits your tire inflation needs and driving preferences.
-

Gaseous State vs Liquid State
Join us on a captivating journey as we unravel the intriguing distinctions between the liquid state and the gaseous state of matter. In the realm of science, these two phases exhibit unique behaviors and properties that play a significant role in our daily lives and numerous industries. At the molecular level, liquids are characterized by closely packed molecules that retain a definite volume while conforming to the shape of their container. Their fluidity and viscosity allow for easy pouring, making them essential in various applications, from quenching your thirst with a refreshing beverage to facilitating chemical reactions in laboratories. On the other hand, gases present a vastly different molecular arrangement, with molecules far apart and exhibiting high kinetic energy. They possess neither a definite volume nor shape and have remarkable compressibility. Gases are the driving force behind numerous industrial processes, energy production, and even the air we breathe. Understanding these disparities is crucial, whether you're a scientist delving into the mysteries of matter or simply curious about the world around you. Join us as we delve into the fascinating "Differences Between Liquid State vs. Gaseous State" and uncover their significance in our everyday existence.
-

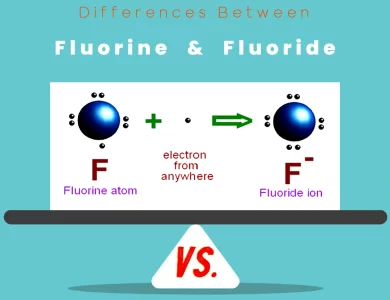
Fluoride vs Fluorine
In the realm of chemistry, the terms "fluorine" and "fluoride" may sound deceptively similar, but their disparities are profound. Fluorine, with its atomic symbol F and atomic number 9, is a fiercely reactive element. It exists as a diatomic gas, rarely found in nature in its elemental form. Its extreme reactivity makes it one of the most formidable elements in the periodic table. In contrast, fluoride is not an element but a chemical compound. It contains the fluoride ion (F⁻) and is formed when fluorine reacts with other elements. Fluoride compounds can be solids, liquids, or gases, depending on their composition. They are notably less reactive than their parent element, making them invaluable in various industries and dental care. The distinctions between these two entities extend beyond their nature. Fluorine, with its pale yellow-green gas and acrid odor, is notoriously toxic and not used directly in most applications. Fluoride compounds, on the other hand, are harnessed for their stability and versatility. They find applications in water treatment, metallurgy, and even toothpaste, where they safeguard our dental health by fortifying tooth enamel. However, the environmental impact of fluoride and the careful regulation of its levels in drinking water remain subjects of ongoing debate and study. To delve deeper into the intriguing world of fluorine and fluoride, read on and explore their diverse roles, properties, and applications.
-

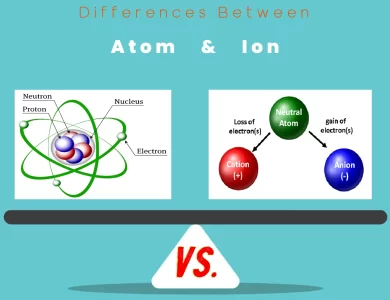
Ion vs Atom
Embark on a journey through the subatomic universe as we unravel the captivating dissimilarities between atoms and ions. Atoms, the fundamental units of matter, and ions, their charged counterparts, are integral to understanding the very essence of our world. Atoms, comprised of protons, neutrons, and electrons, stand as the elemental building blocks that compose all matter. In contrast, ions emerge when atoms gain or lose electrons, leading to a charge imbalance and distinctive properties. These disparities in charge and composition usher in a host of differences in behavior, size, and behavior during chemical reactions. The quantum behaviors of these entities also diverge, with atoms abiding by specific energy levels and orbitals, while ions exhibit altered electron distributions. Moreover, their roles extend beyond the microscopic realm, shaping everything from the materials around us to the batteries that power our devices. From the intricacies of atomic structures to the allure of ionic interactions, this exploration sheds light on the captivating distinctions that underpin the subatomic world. Join us on this cosmic journey as we navigate through the realm of atoms and ions, unraveling their intricacies and uncovering their significance in the grand tapestry of the universe.
-

Inorganic Substances vs Organic
Delving into the intricate world of chemistry, we uncover the defining differences between organic and inorganic substances. Organic compounds, centered around carbon-based molecules, constitute the building blocks of life and intricate structures found in living organisms. On the other hand, inorganic substances, lacking the carbon-carbon bond, span a diverse spectrum that includes metals, minerals, and salts, each with distinct properties and applications. As we navigate these two captivating realms, we unveil the versatile properties of organic compounds, shaped by functional groups and complex molecular structures. In contrast, the stability and unique properties of inorganic compounds, often harnessed in electronics and materials science, shine through. The interplay of organic and inorganic chemistry enriches fields like medicine, renewable energy, and sustainability, underscoring their profound impact on modern life. Join us on this chemical journey to uncover the fascinating contrasts and collaborations between organic and inorganic substances, shedding light on the complexities that shape our world.
-

Inorganic Chemistry vs Organic Chemistry
Embark on a fascinating journey through the realms of Organic Chemistry and Inorganic Chemistry. These branches of chemistry, while sharing foundational principles, diverge into distinct territories with unique characteristics. Organic Chemistry delves into the world of carbon compounds, exploring their intricate structures, diverse functional groups, and reactions that power life itself. In contrast, Inorganic Chemistry expands the horizon, encompassing compounds beyond carbon – metals, minerals, and their complex behaviors. The bond between atoms takes different forms in these realms. Organic Chemistry is marked by covalent bonding and the interplay of functional groups, while Inorganic Chemistry delves into both covalent and ionic bonding, often accentuated by coordination bonds with metal atoms. These differences in bonding patterns give rise to varying reactivity and applications. Organic Chemistry finds its applications in pharmaceuticals, food, textiles, and plastics, impacting our daily lives. On the other hand, Inorganic Chemistry's influence is felt in catalysis, materials science, and even the development of advanced electronics. Whether you're intrigued by the intricacies of organic reactions or the mysteries of metal-driven behaviors, this exploration of differences between Organic Chemistry and Inorganic Chemistry promises to unveil the captivating tapestry of the molecular world.
-

Vapor vs Gas
Delving into the realms of matter, the disparities between gas and vapor emerge as a captivating exploration of their distinctive properties and behaviors. Gas, characterized by its molecules' rapid and uninhibited motion, contrasts with vapor's equilibrium-seeking nature. Gases, like oxygen and nitrogen, inhabit the Earth's atmosphere, shaping the air we breathe, while vapors, such as steam from boiling water, create ephemeral clouds and contribute to the intricate water cycle. The visible mist above a cup of hot tea or the evocative scent diffusing through a room highlights vapor's mesmerizing dance between different phases. The interplay of energy absorption and release sets these entities apart – gases often release energy during condensation, while vaporization demands an input of energy to transition from a liquid or solid state. Intermolecular forces play a pivotal role, with gases experiencing weaker Van der Waals forces compared to vapors that can exhibit hydrogen bonding. This exploration extends into the world of industry and applications, where gases find utility in diverse industrial processes, and vapors showcase their culinary prowess in steaming and cooking techniques. By understanding the nuanced differences between gas and vapor, we unlock a deeper appreciation for the intricate tapestry of matter that shapes our environment and daily experiences.
-

Vapor vs Steam
Journey into the captivating world of steam and vapor as we unravel their intriguing differences. While often used interchangeably, these terms hold distinct meanings that shape various aspects of our lives. Steam, the gaseous form of water formed by heating it to its boiling point, has powered industries, generated electricity, and even revolutionized cleaning methods. On the other hand, vapor encompasses the broader realm of substances transitioning from liquid or solid states to gases, adding aroma to beverages, driving medical inhalation therapies, and creating mystique through processes like sublimation. Beyond their scientific implications, steam and vapor have seeped into our culture, inspiring literary genres like steampunk and symbolizing the ephemeral in storytelling. But the contrasts don't stop there—steam's environmental impact, rooted in its energy generation methods, differs from the considerations surrounding vapor and its emissions during certain processes. As we delve into the nuances of these entities, a richer understanding of their roles in everyday life, industries, and artistic expressions emerges. Join us in the exploration of steam and vapor, where science meets symbolism and innovation intertwines with imagination.
-

Atoms vs Elements
Embark on a captivating journey through the intricate tapestry of chemistry as we unravel the fundamental disparities between elements and atoms. Imagine elements as nature's distinct characters, each with its own set of traits and properties. Composed of identical atoms, elements represent the building blocks of matter, creating the diverse substances that form the world around us. On the other hand, atoms, the tiniest constituents of elements, comprise a nucleus housing protons and neutrons, encircled by electrons dancing in energy levels. These atoms, resembling cosmic dancers, orchestrate the chemistry of elements, forming bonds, participating in reactions, and giving rise to compounds with a remarkable array of properties. From their behavior in chemical reactions to their roles in the periodic table, elements and atoms play distinct yet interconnected roles in the intricate dance of chemistry. While elements showcase their diversity on the periodic table's canvas, atoms bring their quantum behaviors to the forefront, guiding the way elements interact and combine. Join us on this voyage of discovery as we navigate the realm of elements and atoms, unearthing the secrets that underpin the composition of matter and the essence of the universe.
- 1
- 2