Organic Chemistry
Here, we delve into the intricate details of this fascinating branch of chemistry, shedding light on the differences in terms and other essential aspects that shape the field. Whether you’re a student, researcher, or simply curious about the subject, this guide is designed to demystify the complexities of Organic Chemistry in a friendly and approachable manner.
Organic Chemistry is the study of carbon-based compounds, which form the basis of life as we know it. Understanding the nuances of this discipline can be challenging, with a vast array of terms and concepts to grasp. In this category, we bring together a wealth of content that explores the differences in terms, definitions, and other crucial elements that are integral to Organic Chemistry. So, let’s embark on this journey together and unravel the mysteries of this captivating field!
-

Oxyacids vs Binary Acids
In the realm of geology, two fundamental processes, cementation and compaction, stand as the cornerstone of sedimentary rock formation. While they share the common objective of transforming loose sediments into solid rock, their mechanisms and impacts diverge significantly. Cementation, akin to nature's adhesive, is a post-depositional process where minerals act as binding agents. These minerals precipitate from mineral-rich solutions, gluing sediment particles together and solidifying them over time. Picture calcite, silica, or iron oxides acting as nature's glue, holding the grains of sand, silt, or clay together. The result? Sedimentary rocks with distinct textures, colors, and porosities, depending on the minerals involved. Compaction, on the other hand, is a physical process intertwined with sediment deposition. It occurs concurrently as layers of sediment accumulate, and the sheer weight of overlying material compresses the sediment below. The particles get squeezed closer together, reducing pore spaces and leading to fine-grained, compacted rocks. Think of it as Earth's slow but persistent sculptor, shaping the sediments into dense, often smooth-textured formations. Understanding these processes is essential for deciphering Earth's history, environmental conditions, and the formation of various sedimentary rock types. So, whether you're a geology enthusiast or a curious mind, delving into the contrasts between cementation and compaction promises a deeper appreciation of our planet's geological wonders.
-

Nucleophile vs Base
In the fascinating world of chemistry, understanding the distinctions between a base and a nucleophile is paramount. Bases, known for their role in acid-base reactions, have the unique ability to accept protons or donate lone pair electrons, thus influencing the pH of a solution. Common examples include hydroxide ions (OH-) and ammonia (NH3). In contrast, nucleophiles, electron-rich entities, are skilled in attacking electrophiles, leading to the formation of new covalent bonds. Prominent nucleophiles encompass chloride ions (Cl-), cyanide ions (CN-), and various amines. These disparities are crucial in comprehending their functions within chemical reactions, making the base vs. nucleophile distinction an essential concept in the realm of chemistry. Whether you're a budding chemist or simply curious about the scientific intricacies of molecules, delving into these differences unveils a world of insights into the dynamic processes that surround us.
-

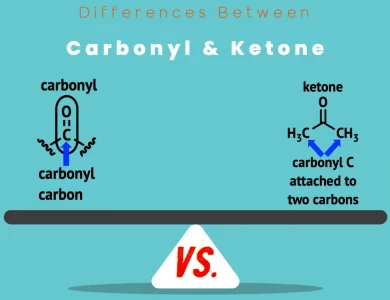
Ketone vs Carbonyl
When it comes to organic chemistry, understanding the nuances between carbonyl compounds and ketones is pivotal. Carbonyl compounds, characterized by a carbon atom double-bonded to an oxygen atom (C=O), encompass a diverse range of molecules, including aldehydes, ketones, carboxylic acids, esters, and amides. In contrast, ketones represent a specific subset within this category, featuring the C(=O) bond situated between two carbon-containing groups. This structural divergence sets the stage for differences in their physical properties, such as boiling and melting points, as well as their reactivity patterns. While both carbonyl compounds and ketones partake in nucleophilic addition reactions, ketones generally exhibit greater resistance to oxidation. This resistance renders ketones invaluable in various chemical processes and industrial applications. To gain a comprehensive understanding of these distinctions, read on to explore the world of carbonyl vs ketone compounds.
-

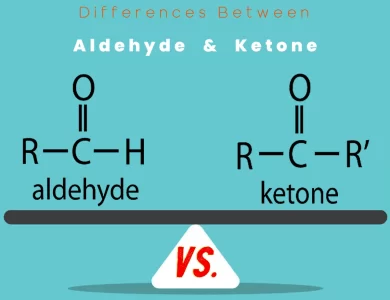
Ketone vs Aldehyde
Delve into the captivating world of organic chemistry as we unravel the intricate disparities between aldehyde and ketone molecules. These two distinct classes of compounds, both featuring the carbonyl functional group, possess unique structural arrangements and reactivity patterns that set them apart. Aldehydes showcase a carbonyl group bonded to a hydrogen atom, while ketones boast a carbonyl group positioned between two carbon atoms. This seemingly subtle difference leads to profound variations in their chemical behavior and applications. In the realm of reactivity, aldehydes are known for their susceptibility to oxidation, readily transforming into carboxylic acids. On the other hand, ketones exhibit a remarkable resistance to oxidation due to the absence of an active hydrogen atom. The role of these compounds extends beyond reactions, touching areas such as fragrances, flavor compounds, solvents, and even pharmaceutical synthesis. Aldehydes find their place in perfumery and preservation, while ketones excel in applications involving solubility, drug development, and materials science. Our comprehensive exploration navigates through the intricacies of aldehydes and ketones, shedding light on their distinct roles in chemistry and industries. Whether you're a chemistry enthusiast, student, or industry professional, understanding these differences offers a deeper appreciation for the intricate dance of molecules that shape our world. Join us as we embark on a journey of discovery, uncovering the fascinating disparities and applications of aldehyde and ketone molecules in our comprehensive blog, "Differences Between Aldehyde vs Ketone."
-

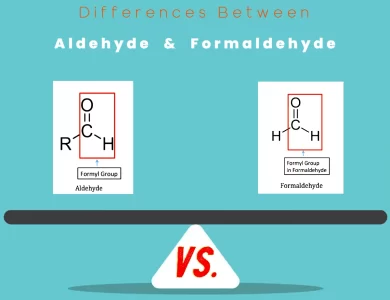
Formaldehyde vs Aldehyde
AspectAldehydeFormaldehydeChemical StructureContains a carbonyl group (C=O) bonded to a carbon and a hydrogen atom.Contains a carbonyl group (H-C=O) bonded to hydrogen and oxygen atoms.Physical StateCan exist as both liquids and gases at room temperature.Exists as a colorless, pungent-smelling gas at room temperature.OdorVaries in odor, from fruity to sharp, depending on the specific compound.Has a distinct pungent and strong odor.Boiling PointBoiling points generally higher than those of corresponding alcohols.Boiling point is relatively low due to its small molecular size.ReactivityParticipates in nucleophilic addition and oxidation reactions.Reacts in nucleophilic addition reactions and can crosslink with proteins.ApplicationsUsed in perfumery, flavoring, pharmaceuticals, and organic synthesis.Employed as a disinfectant, preservative, and in the production of materials.Biochemical RolePlays a role in metabolism and signaling pathways within living organisms.Not a significant participant in biological processes.Polymer ChemistryLimited role in polymer chemistry compared to other functional groups.Essential for the production of formaldehyde-based resins used in construction and materials manufacturing.Health ImpactGenerally safe in small amounts but can cause irritation or allergies in some individuals.Prolonged exposure can lead to respiratory irritation, sensitization, and health issues.Environmental ImpactCan contribute to air pollution when emitted as pollutants.Formaldehyde emissions can contribute to air pollution and ground-level ozone formation.Analytical TechniquesDetected and quantified using gas chromatography, HPLC, and …
-

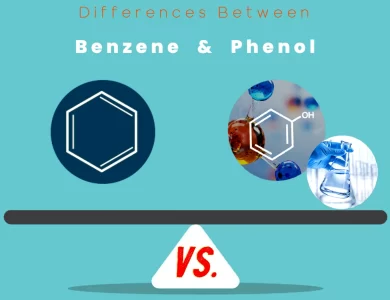
Phenol vs Benzene
Delving into the realm of organic chemistry, the disparities between benzene and phenol come to life as two aromatic compounds take center stage. Benzene, known for its resonance-driven stability, boasts a hexagonal ring structure intertwined with alternating single and double bonds. In contrast, phenol adds a twist to the narrative with a hydroxyl group adorning its aromatic ring, enhancing reactivity and versatility. While benzene leans towards electrophilic aromatic substitutions, phenol's hydroxyl group invites both substitution and addition reactions into its chemistry. Beyond reactivity, these compounds unveil divergent physical properties. Benzene exhibits a lower boiling point influenced by London dispersion forces, while phenol's hydrogen bonding elevates its boiling point. The applications of benzene span industries like petrochemicals and pharmaceuticals, while phenol finds its niche in antiseptics, plastics, and resins. Despite their aromatic commonality, their impacts diverge—benzene, a carcinogen with environmental concerns, and phenol, a safer alternative with less toxicity. The differences between benzene and phenol are more than skin deep; they reflect the intricacies of molecular arrangement and chemical behavior. This exploration unravels their unique stories, highlighting their roles in the intricate tapestry of chemistry and inspiring curiosity about the diverse world of compounds.
-

Ethanol vs Ethane
Embark on a journey of discovery as we delve into the intriguing disparities between two prominent chemical compounds: ethane and ethanol. These distinct entities, while sharing some similarities in their chemical makeup, exhibit remarkable differences that set them apart in various facets. Ethane, a simple hydrocarbon gas, finds its significance as both a fuel and a petrochemical feedstock, propelling industries and powering our modern world. On the other hand, ethanol, an alcohol compound with a characteristic odor, boasts a diverse range of applications, from alcoholic beverages to pharmaceuticals and even sustainable biofuels. The dissimilarities extend to their physical states; ethane exists as an odorless gas, while ethanol presents itself as a clear, aromatic liquid. Their boiling and melting points diverge significantly due to their unique molecular structures. Additionally, these compounds vary in their reactivity, environmental impact, and economic significance. Ethane's combustion emissions contribute to its role in global warming, while ethanol's renewable nature positions it as a greener alternative.
-

Methanol vs Ethanol
Delve into the intriguing world of organic alcohols as we unravel the differences between ethanol and methanol. While these two compounds share certain similarities, their unique chemical compositions and diverse applications unveil a tapestry of contrasts. Ethanol, known for its role in alcoholic beverages and biofuel additives, stands apart from methanol, often used in chemical synthesis and industrial processes. However, it's not just their applications that differ – their physical properties, environmental impacts, and health considerations diverge significantly. Dive into this captivating exploration to gain a comprehensive understanding of the roles these alcohols play in our lives and industries. Whether you're a chemistry enthusiast or simply curious about the molecules that shape our world, this journey promises to enlighten and engage.
-

Phenyl vs Phenol
Embarking on a scientific journey, one often encounters compounds that share similar roots yet harbor profound differences. Such is the case with phenol and phenyl – two terms that intrigue the chemically curious. Phenol, an organic compound, flaunts a hydroxyl group attached to a benzene ring, endowing it with solubility, acidity, and reactivity. It finds its place in industrial chemistry, from plastics to disinfectants, and even in medical applications. Phenyl, on the other hand, is not a standalone entity but a fragment, derived from a benzene ring by removing a hydrogen atom. Its nonpolar and lipophilic nature grants it significance in drug design and agrochemical enhancement.
-

Phenols vs Alcohols
Delve into the captivating world of organic chemistry as we unravel the intriguing disparities between alcohols and phenols. While both compounds feature a hydroxyl (-OH) group, their structural arrangements unveil a realm of differences. Alcohols, characterized by the hydroxyl group attached to a carbon atom, play versatile roles as solvents, disinfectants, and pharmaceutical components. On the other hand, phenols showcase enhanced acidity due to their hydroxyl group's direct bond with an aromatic ring, often a benzene structure. This distinction leads to divergent reactivity patterns, making phenols ideal for electrophilic aromatic substitution reactions. From their applications in antiseptics, adhesives, and plastics to potential health benefits, phenolic compounds offer a spectrum of possibilities. Our comprehensive guide navigates through their varied properties, safety considerations, and environmental impact. Whether you're a chemistry enthusiast, a researcher, or simply curious about the molecules shaping our world, join us on a journey to uncover the captivating differences between alcohols and phenols.
- 1
- 2