Physical Chemistry
Here, we embark on a journey to explore the diverse array of terms, concepts, and phenomena that define this enthralling branch of science. Prepare to dive deep into the realm of matter, energy, and their interactions as we uncover the differences that make Physical Chemistry a fascinating subject.
-

Fire Point vs Flash Point
In the realm of fire safety and the handling of flammable substances, understanding the differences between Flash Point and Fire Point is paramount. These two terms are often used interchangeably, but they serve distinct purposes in assessing the flammability of materials. Flash Point is the temperature at which a liquid substance produces enough vapor to ignite when exposed to an open flame or spark. It essentially marks the ignition threshold and is crucial for industries involved in the transportation, storage, and handling of flammable liquids. Knowing the flash point of a substance helps prevent accidents, guides safe storage practices, and ensures compliance with safety regulations. On the other hand, Fire Point represents the temperature at which a liquid substance can sustain combustion once ignited. It goes beyond the initial ignition and tells us whether a fire will continue to burn without the need for an external ignition source. Fire point is particularly significant in assessing the severity of a fire hazard and planning effective firefighting strategies and emergency response protocols. To gain a comprehensive understanding of these critical parameters, it's essential to delve into their testing methods, regulatory implications, and their roles in various industries. By recognizing the distinctions between flash point and fire point, industries can enhance safety measures, mitigate fire risks, and ensure the secure handling of flammable materials.
-

Heat vs Work
Work and heat, though both terms closely related to energy transfer, stand as distinctive concepts in the realm of physics and thermodynamics. These fundamental principles are integral to understanding the flow of energy in various systems and processes. Work, often associated with the application of force, embodies the transfer of mechanical energy leading to the displacement of an object. Its measurement unit, the joule (J), quantifies the extent of this energy transfer. Work's directionality further categorizes it as positive, negative, or zero, dependent on the alignment of force with motion. This concept finds practical application in everything from lifting objects to driving machines. In contrast, heat represents the transfer of thermal energy arising from temperature differences. Like work, it is quantified in joules (J) and finds utility in everyday life, from cooking to temperature regulation. Heat's remarkable feature is its lack of directional component—instead, it focuses solely on the transfer of internal energy through conduction, convection, and radiation. These disparities culminate in a deeper understanding of the First Law of Thermodynamics, which emphasizes energy conservation. Work and heat play pivotal roles in this law, showcasing their relevance in the world of physics and engineering. As we explore the intricate differences between these energy-transfer mechanisms, we gain insight into the forces that shape our physical universe.
-

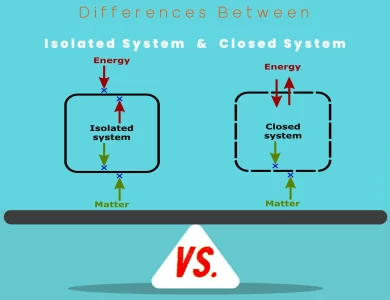
Closed System vs Isolated System
In the realm of physics and thermodynamics, two fundamental concepts—isolated systems and closed systems—play a pivotal role in understanding how matter and energy interact within physical boundaries. The primary disparity between these two lies in their treatment of energy exchange. Isolated systems, often encountered in theoretical physics, are characterized by their strict conservation of both matter and energy. These systems do not permit any matter or energy exchange with the surrounding environment, making them valuable for theoretical studies and idealized models. In contrast, closed systems, commonly encountered in practical applications, allow energy transfer across their boundaries while preserving a constant mass. This flexibility makes them indispensable for understanding real-world processes like chemical reactions, engineering systems, and environmental phenomena, where energy interactions play a vital role. By exploring the nuances of isolated and closed systems, scientists, engineers, and researchers can make informed decisions on which framework to apply based on the specific goals and nature of the system under study. This knowledge is crucial for both theoretical explorations of fundamental principles and the optimization of practical processes in various fields.
-

Cathode vs Anode
In the realm of electrochemistry, the dichotomy between anode and cathode forms the foundation of diverse applications. Anode and cathode are fundamental components in electrochemical reactions, showcasing contrasting roles that define their contributions. Anode, the site of oxidation, bids adieu to electrons, while cathode, the realm of reduction, embraces them with open arms. The electron flow direction sets them apart – anode sees electrons exiting into the external circuit, whereas cathode welcomes electrons from the circuit. This division of labor extends to their functions as well. Anode, often an electron donor, finds purpose in energy storage systems, sacrificial protection mechanisms, and electroplating processes. On the flip side, cathode, as an electron acceptor, takes center stage in rechargeable batteries, electroplating applications, and cathodic protection strategies against corrosion. Their dynamic interplay powers batteries, enhances surface finishes, and safeguards vital structures. Whether you're navigating the intricacies of batteries or unraveling the magic of electroplating, understanding the unique contributions of anode and cathode is essential. This exploration into their differences unveils the captivating world of electrochemical reactions, shedding light on their diverse roles, functions, and applications.
-

Non-Metals vs Metals
Welcome to a captivating journey through the captivating realms of metals and non-metals. In this blog, we'll uncover the secrets behind these elemental entities and explore their unique properties, applications, and significance in our everyday lives. From the shiny brilliance of metals to the versatile characteristics of non-metals, we'll delve into their distinct attributes that shape our physical world. Metals, with their characteristic metallic luster, exceptional strength, and conductivity, find applications in construction, transportation, electronics, and jewelry. Meanwhile, non-metals, which lack the metallic luster, exhibit diverse properties and play vital roles in healthcare, energy production, agriculture, and beyond. We'll delve into their contrasting physical and chemical properties, from malleability and ductility to their reactivity and bonding characteristics. Join us on this captivating adventure as we explore the interplay between metals and non-metals, their impact on our environment, and the importance of sustainable practices. We'll unravel the mysteries behind their extraction, production, and disposal, while highlighting the efforts being made to minimize their environmental footprint. By the end of this blog, you'll gain a deeper understanding of how metals and non-metals shape our world and the steps we can take to foster a more sustainable future. So, get ready to embark on a journey through the wonders of metals and non-metals, where shimmering brilliance and versatile properties await. Let's delve into the captivating world of elements and expand our knowledge of these fundamental components that surround us.