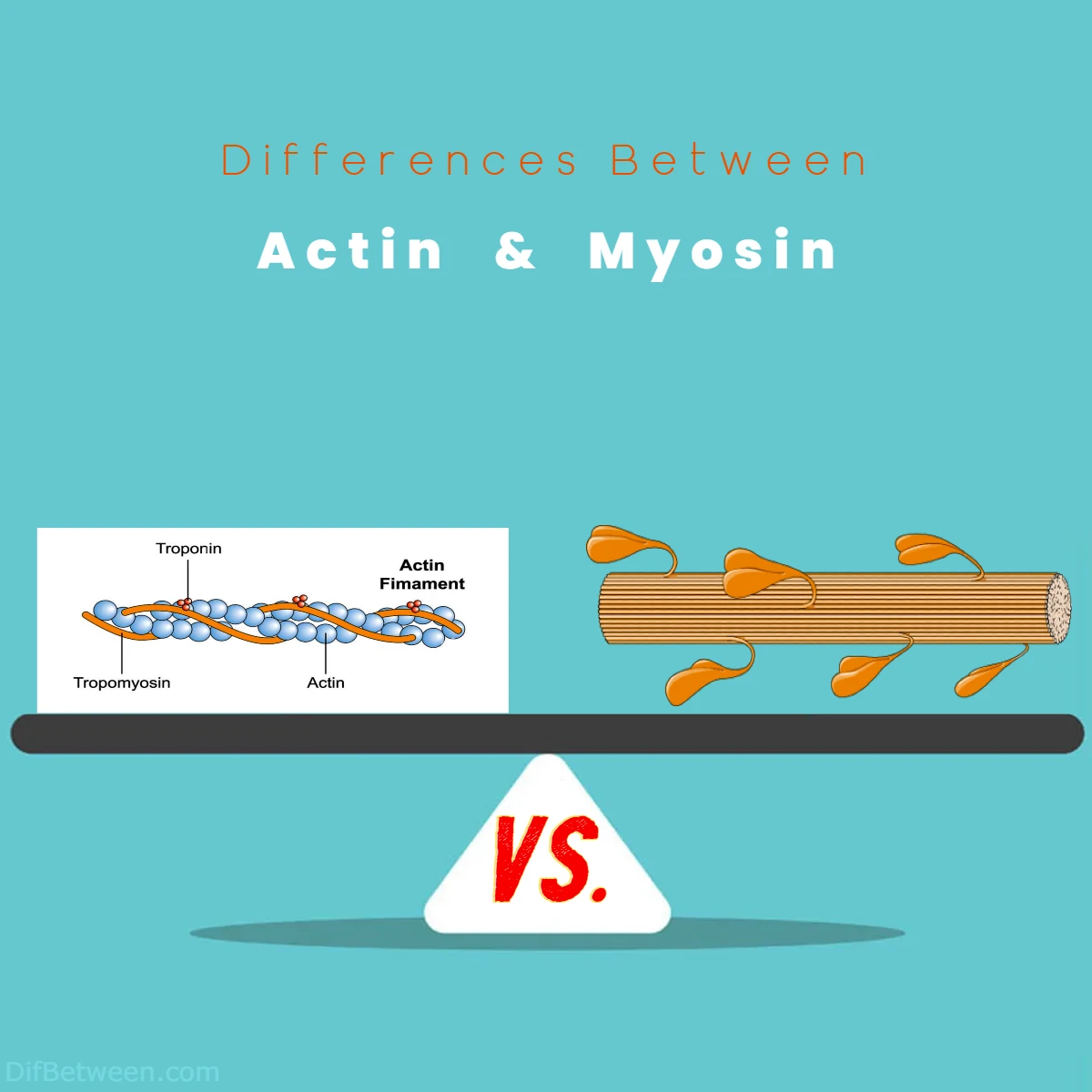
The main differences between Actin and Myosin lie in their structural characteristics and vital roles within the cellular and physiological processes. Actin, a thin, globular protein, primarily serves as a cytoskeletal framework and is involved in a wide array of cellular functions, from cell shape maintenance to immune cell migration. On the other hand, Myosin, a thick, multi-chain protein with myosin heads, is the workhorse behind mechanical force generation, driving muscle contraction, cell motility, and intracellular transport. These distinctions in structure and function make Actin and Myosin integral players in the intricate choreography of life, where Actin provides the stage, and Myosin performs the energetic dance, harmonizing to create the magic of movement.
| Aspect | Actin | Myosin |
|---|---|---|
| Structure | Thin, globular protein | Thick, multi-chain protein with myosin head |
| Function | Cytoskeletal support, cell motility, cell division | Mechanical force generation, muscle contraction, cell motility, intracellular transport |
| Regulation of Activity | Controlled via actin-binding proteins, Rho GTPases | Phosphorylation of myosin head, calcium ion concentration |
| Cellular Localization | Ubiquitous, cytoskeleton, cell cortex, microvilli | Sarcomeres in muscle cells, various locations in non-muscle cells, organelles |
| Energy Utilization | Indirect (no direct energy consumption) | Direct (ATP hydrolysis for force generation) |
| Role in Muscle Contraction | Provides Actin filaments | Provides Myosin filaments |
| Involvement in Cell Motility | Formation of cellular protrusions, immune cell migration, cytokinesis | Cell crawling, muscle cell contraction, cytoplasmic streaming, intracellular transport |
| Genetic Variants | Multiple isoforms with distinct functions | Multiple isoforms tailored for muscle, non-muscle, and smooth muscle |
| Interactions with Other Proteins | Actin-binding proteins, Myosin, tropomyosin-troponin complex | Actin, Myosin Light Chain Kinase, Myosin Light Chain Phosphatase |
| Role in Diseases | Cardiomyopathies, cytoskeletal disorders, cancer metastasis | Myosin myopathies, hypertrophic cardiomyopathy, neurological disorders, cancer metastasis |
| Evolutionary Aspects | Highly conserved across diverse organisms | Highly conserved with greater diversity in isoforms |
| Role in Non-Muscle Cells | Maintains cell shape, cell motility, endocytosis, exocytosis | Cell motility, intracellular transport, cytokinesis, cell adhesion |
| Role in Muscle Types | Primarily involved in thin filaments in sarcomeres | Forms thick filaments in sarcomeres, various isoforms for different muscle types |
| Significance in Drug Development | Less common targets in drug development | Gaining interest, especially in cardiac medications |
| Interplay in Muscle Contraction | Provides structure for muscle contraction | Generates mechanical force for muscle contraction |
| Implications in Biotechnology | Used in microscopy, cell culture | Utilized in in vitro motility assays, drug screening |
| Role in Aging | Impacts cell structure and function | Associated with age-related muscle changes |
| Clinical Applications | Less frequently targeted in therapies | Used in cardiac medications and muscle-related treatments |
| Beyond Muscle Contraction | Participates in a wide range of cellular processes | Diverse functions beyond muscle contraction |
| Involvement in Cardiac Health | Associated with genetic cardiomyopathies | Linked to hypertrophic cardiomyopathy, explored in heart medications |
Actin, often referred to as the “thin filament,” and Myosin, the “thick filament,” are like dynamic dance partners, working in perfect harmony to bring about the magic of movement. While Actin serves as the structural scaffold and plays a role in a myriad of cellular processes, Myosin steals the spotlight as the powerhouse generating the mechanical force behind muscle contraction and cellular motion.
Differences Between Actin and Myosin
Structure and Composition
Actin:
Actin, often referred to as “thin filaments,” is a protein that plays a pivotal role in muscle contraction and various cellular processes. Structurally, Actin is a globular protein composed of monomers known as G-actin (globular actin). These G-actin monomers polymerize to form long, filamentous structures called F-actin (filamentous actin). The polymerization of G-actin into F-actin is a reversible process that can be regulated within cells.
Actin filaments are typically about 7 nanometers in diameter and consist of two strands of F-actin coiled around each other. These filaments provide structural support to the cell’s cytoskeleton and are involved in various cellular functions, such as cell division, cell shape maintenance, and cell motility.

Myosin:
Myosin, on the other hand, is often referred to as “thick filaments.” Structurally, Myosin is a motor protein composed of multiple polypeptide chains, with the primary chain known as the heavy chain. These heavy chains contain regions responsible for binding to Actin and for hydrolyzing adenosine triphosphate (ATP) to generate mechanical force.
Myosin molecules are larger and more complex compared to Actin, with a characteristic structure known as the myosin head. The myosin head contains an ATP-binding site and an Actin-binding site. It is this head that undergoes conformational changes during muscle contraction, resulting in the sliding of Actin and Myosin filaments and the generation of force.
In summary, the primary structural difference between Actin and Myosin is that Actin is a thin, globular protein, while Myosin is a thick, multi-chain protein with a distinctive myosin head.

Function
Actin:
Actin is involved in several crucial functions within the cell:
- Muscle Contraction: In muscle cells, Actin, together with Myosin, is responsible for the contraction of muscle fibers. During contraction, Actin filaments slide over Myosin filaments, shortening the muscle.
- Cytoskeleton Support: Actin filaments form the backbone of the cell’s cytoskeleton, providing structural support and maintaining cell shape.
- Cell Motility: Actin is essential for cell motility in various cell types, including immune cells, which need to move to infection sites.
- Cell Division: Actin plays a role in cytokinesis, the process by which a single cell divides into two daughter cells.
- Cell Signaling: Actin is also involved in cell signaling pathways, helping to transduce extracellular signals into intracellular responses.
Myosin:
Myosin’s primary function is to generate mechanical force and movement:
- Muscle Contraction: In muscle cells, Myosin interacts with Actin to produce the mechanical force required for muscle contraction. Myosin heads attach to Actin and undergo a power stroke when stimulated by ATP, leading to the sliding of Actin and Myosin filaments.
- Cell Motility: Myosin is involved in the movement of cells, including the migration of immune cells to sites of infection and the transportation of organelles within cells.
- Intracellular Transport: Myosin motors are responsible for transporting various cargoes within cells, such as vesicles, along the cytoskeleton’s tracks.
- Maintenance of Cell Shape: Myosin, particularly in non-muscle cells, plays a role in maintaining cell shape and integrity.
In summary, while both Actin and Myosin play vital roles in muscle contraction and cell motility, Actin has broader functions in maintaining cell structure and participating in signaling pathways, while Myosin is primarily focused on generating mechanical force and facilitating movement.
Regulation of Activity
Actin:
Actin’s activity can be regulated through several mechanisms:
- Tropomyosin-Troponin Complex: In muscle cells, the binding of calcium ions to troponin, a component of the tropomyosin-troponin complex, allows Actin and Myosin to interact, leading to muscle contraction.
- Actin-Binding Proteins: Various Actin-binding proteins can modulate Actin’s polymerization and depolymerization, affecting the formation of Actin filaments.
- Rho GTPases: Small GTPases, like Rho, can regulate Actin dynamics by controlling the assembly and disassembly of Actin filaments.
Myosin:
Myosin’s activity is regulated primarily through phosphorylation of the myosin head:
- Myosin Light Chain Kinase (MLCK): Phosphorylation of the myosin head by MLCK activates the myosin motor, allowing it to bind to Actin and generate force. This is a key step in muscle contraction.
- Myosin Light Chain Phosphatase (MLCP): Dephosphorylation of the myosin head by MLCP deactivates the myosin motor, leading to muscle relaxation.
- Calcium Ion Concentration: Myosin activity in muscle cells is also regulated by the concentration of calcium ions, which activate MLCK and, consequently, myosin phosphorylation.
In summary, the regulation of Actin and Myosin involves intricate biochemical processes, with Actin’s regulation often focusing on its polymerization and depolymerization, while Myosin’s regulation centers on the phosphorylation of its myosin head.
Cellular Localization
Actin:
Actin is a highly abundant protein found throughout the cell:
- Cytoskeleton: Actin filaments are a major component of the cytoskeleton, providing mechanical support to the cell and aiding in cell shape maintenance.
- Cell Cortex: Actin is particularly concentrated in the cell cortex, a region just beneath the cell membrane, where it forms a meshwork that influences cell shape and motility.
- Microvilli: Actin is present in the microvilli of epithelial cells, where it contributes to the maintenance of these finger-like projections.
- Filopodia and Lamellipodia: Actin plays a role in the formation of filopodia (thin, spike-like projections) and lamellipodia (flat, sheet-like extensions) in migrating cells.
Myosin:
Myosin is typically localized in specific regions within the cell:
- Muscle Cells: In muscle cells, Myosin is abundant and concentrated in the sarcomeres, the repeating contractile units responsible for muscle contraction.
- Non-Muscle Cells: In non-muscle cells, Myosin can be found in various cellular locations, including the cell cortex, stress fibers, and the leading edge of migrating cells.
- Organelles: Certain types of Myosin, known as myosin motors, are associated with organelles and vesicles, facilitating their intracellular transport.
In summary, Actin is ubiquitously distributed throughout the cell, with a strong presence in the cytoskeleton, whereas Myosin’s localization varies depending on cell type and function, with a prominent role in muscle cells.
Energy Utilization
Actin:
Actin does not directly consume energy during its role in muscle contraction or cytoskeletal support. Instead, the energy required for Actin’s functions is provided indirectly through the hydrolysis of ATP by Myosin. Actin itself does not participate in ATP hydrolysis, making it an energy-efficient component of the muscle and cytoskeleton.
Myosin:
Myosin is an energy-consuming protein. It hydrolyzes ATP to generate the mechanical force required for muscle contraction and various cellular movements. The energy derived from ATP hydrolysis powers the conformational changes in the myosin head, allowing it to bind to Actin, perform the power stroke, and generate movement.
In summary, Actin is not directly involved in energy consumption, as Myosin is the energy-consuming partner in the process of muscle contraction and cell motility.
Role in Muscle Contraction
Actin:
In the context of muscle contraction, Actin’s role is as follows:
- Thin Filaments: Actin is a component of the thin filaments in the sarcomeres of muscle cells. These filaments slide over the thick Myosin filaments, resulting in muscle shortening and contraction.
- Active Sites: Actin exposes binding sites for Myosin heads, allowing the Myosin heads to attach during muscle contraction.
Myosin:
In muscle contraction, Myosin plays a crucial role:
- Thick Filaments: Myosin forms the thick filaments in the sarcomeres of muscle cells. These filaments contain myosin heads that interact with Actin and produce the mechanical force needed for contraction.
- Cross-Bridge Cycling: Myosin heads bind to Actin, undergo a power stroke powered by ATP hydrolysis, detach, and then repeat the process in a cyclic manner, leading to Actin-Myosin filament sliding and muscle contraction.
In summary, Actin and Myosin act in tandem during muscle contraction, with Actin providing the binding sites for Myosin and Myosin generating the force required for muscle shortening.
Involvement in Cell Motility
Actin:
Actin is a key player in various forms of cell motility:
- Crawling and Migration: Actin is involved in the formation of cellular protrusions like lamellipodia and filopodia, which assist in cell crawling and migration.
- Immune Cell Movement: Immune cells, such as leukocytes, rely on Actin to migrate toward sites of infection, enabling the immune response.
- Cytokinesis: Actin participates in cytokinesis, the process of cell division, by forming a contractile ring that pinches the cell in two.
Myosin:
Myosin is instrumental in cellular movements as well:
- Muscle Cell Contraction: In muscle cells, Myosin enables the contractile movement, such as flexing your biceps or pumping blood from your heart.
- Cytoplasmic Streaming: Myosin is responsible for the movement of cytoplasm within plant cells, a process known as cytoplasmic streaming.
- Intracellular Transport: Myosin motors transport organelles and vesicles along the cytoskeleton, facilitating their movement within the cell.
In summary, both Actin and Myosin are vital for cell motility, with Actin primarily involved in cell crawling, immune cell movement, and cytokinesis, while Myosin drives muscle cell contraction, cytoplasmic streaming, and intracellular transport.
Genetic Variants
Actin:
Actin is encoded by a family of genes in various organisms. In humans, there are six different Actin isoforms, each encoded by a separate gene. These isoforms are broadly categorized as α-Actin (found in muscle tissues) and β-Actin (found in non-muscle tissues). The genetic variants among Actin isoforms give rise to specialized forms of Actin tailored for specific cellular functions.
Myosin:
Myosin is encoded by multiple genes, resulting in various isoforms with distinct functions:
- Muscle Myosin: Muscle cells express specific Myosin isoforms, such as myosin heavy chain (MHC), that are responsible for muscle contraction.
- Non-Muscle Myosin: Non-muscle cells express different Myosin isoforms that are involved in various cellular processes, including cell motility, cytokinesis, and intracellular transport.
- Smooth Muscle Myosin: Smooth muscle cells contain their own specialized Myosin isoforms tailored for the unique properties of smooth muscle tissue.
In summary, the genetic diversity of Myosin isoforms allows for specialization in function, with distinct isoforms present in muscle, non-muscle, and smooth muscle cells.
Interactions with Other Proteins
Actin:
Actin interacts with various proteins to modulate its functions:
- Myosin: Actin and Myosin directly interact during muscle contraction and cell motility, forming the basis of the sliding filament theory.
- Tropomyosin-Troponin Complex: In muscle cells, the tropomyosin-troponin complex regulates Actin’s interaction with Myosin.
- Actin-Binding Proteins: Proteins like profilin and cofilin regulate Actin’s polymerization and depolymerization, influencing its role in cytoskeletal dynamics.
Myosin:
Myosin interacts with several proteins to facilitate its function:
- Actin: Myosin binds to Actin during muscle contraction and cell motility, forming the cross-bridges that produce force.
- Myosin Light Chain Kinase (MLCK): MLCK phosphorylates Myosin’s light chains, activating the myosin motor.
- Myosin Light Chain Phosphatase (MLCP): MLCP dephosphorylates Myosin’s light chains, deactivating the myosin motor.
In summary, Actin and Myosin both have specific interacting partners that modulate their functions. Actin primarily interacts with Myosin and Actin-binding proteins, while Myosin interacts with Actin and regulatory enzymes like MLCK and MLCP.
Role in Diseases
Actin:
Actin is associated with several diseases and conditions:
- Cardiomyopathies: Mutations in Actin genes can lead to various forms of cardiomyopathy, a group of heart muscle disorders.
- Cytoskeletal Disorders: Defects in Actin can result in cytoskeletal disorders, leading to conditions like muscular dystrophy and Charcot-Marie-Tooth disease.
- Cancer Metastasis: Actin’s role in cell motility is implicated in cancer metastasis, where tumor cells migrate and invade other tissues.
- Cell Migration Disorders: Actin dysfunction can lead to impaired immune cell migration, contributing to immunodeficiency disorders.
Myosin:
Myosin is also linked to various diseases and conditions:
- Myosin Myopathies: Mutations in Myosin genes can cause congenital myopathies, a group of muscle disorders characterized by muscle weakness and abnormal muscle function.
- Hypertrophic Cardiomyopathy: Genetic mutations in Myosin genes are associated with hypertrophic cardiomyopathy, a condition in which the heart’s muscle becomes abnormally thickened.
- Neurological Disorders: Some forms of Myosin are expressed in neuronal tissues and play a role in neuronal development and function. Mutations in these genes can lead to neurological disorders.
- Cancer Metastasis: Myosin is implicated in cancer metastasis as well, as it contributes to the motility of cancer cells.
In summary, both Actin and Myosin are linked to a range of diseases, primarily those affecting muscles and the heart, but also neurological and immune system disorders, as well as their roles in cancer metastasis.
Evolutionary Aspects
Actin:
Actin is one of the most highly conserved proteins in evolution, meaning that its structure and function have remained relatively unchanged across a wide range of organisms over millions of years. The conservation of Actin highlights its fundamental role in cellular processes. Actin is found in nearly all eukaryotic cells, from simple single-celled organisms to complex multicellular organisms.
Myosin:
Myosin is also a highly conserved protein, but it exhibits greater diversity in terms of its isoforms and functions. Myosin isoforms have evolved to perform specialized roles in different cell types. For example, muscle Myosin is distinct from non-muscle Myosin, reflecting the specialization of Myosin in muscle contraction and various cellular movements.
In summary, Actin is more universally conserved in terms of its structure and function, whereas Myosin exhibits greater diversity in function due to the evolution of distinct isoforms for specific tasks.
Role in Non-Muscle Cells
Actin:
Actin is ubiquitous in non-muscle cells and plays diverse roles:
- Cell Shape Maintenance: Actin filaments, particularly in the cell cortex, help maintain cell shape and integrity.
- Cell Motility: Actin is vital for cell crawling and migration, contributing to processes like wound healing and tissue repair.
- Endocytosis and Exocytosis: Actin participates in the processes of endocytosis (cellular uptake of substances) and exocytosis (release of substances), aiding in vesicle movement and membrane remodeling.
- Neuronal Function: In neurons, Actin is involved in synaptic plasticity, which is essential for learning and memory.
Myosin:
In non-muscle cells, Myosin has a broad array of functions:
- Cell Motility: Myosin motors are involved in cell crawling, migration, and the formation of cellular protrusions like lamellipodia and filopodia.
- Intracellular Transport: Myosin motors transport organelles, vesicles, and other cargoes within cells.
- Cytokinesis: Myosin plays a crucial role in the contractile ring formation during cytokinesis, enabling cell division.
- Cell Adhesion: Some Myosin isoforms are linked to cell adhesion processes, including the regulation of focal adhesions.
In summary, both Actin and Myosin contribute significantly to the dynamics and functions of non-muscle cells, participating in processes like cell shape maintenance, motility, endocytosis, exocytosis, intracellular transport, and cell division.
Role in Muscle Types
Actin:
Actin is primarily associated with muscle cells, where it contributes to muscle contraction. In muscle tissue, there are two main types of Actin filaments:
- Thin Filaments: Actin forms the thin filaments in sarcomeres, which are the contractile units of skeletal and cardiac muscle.
- Tropomyosin-Troponin Regulation: Actin in muscle cells is regulated by the tropomyosin-troponin complex, which controls Actin’s interaction with Myosin during contraction.
Myosin:
Myosin is also intimately tied to muscle function:
- Thick Filaments: Myosin forms the thick filaments in sarcomeres, and the myosin heads interact with Actin to produce muscle contraction.
- Cross-Bridge Cycling: Myosin heads participate in a cyclic cross-bridge cycle, binding to Actin, undergoing a power stroke, and generating force during muscle contraction.
- Muscle Fiber Types: Different Myosin isoforms are found in various muscle fiber types, contributing to differences in muscle function and contractile properties.
In summary, Actin and Myosin are essential for muscle contraction, with Actin forming the thin filaments and Myosin forming the thick filaments in sarcomeres.
Significance in Drug Development
Actin:
Actin is less frequently targeted in drug development, as its essential functions in cellular processes make it challenging to selectively inhibit. However, research into Actin-targeting drugs continues in areas like cancer therapy, where limiting cell motility can hinder metastasis.
Myosin:
Myosin has gained attention in drug development, particularly in the field of cardiac medications. Drugs that influence Myosin function, such as myosin activators or myosin inhibitors, are being explored to treat conditions like heart failure.
In summary, while Actin has essential functions in cellular processes, Myosin is of greater interest in drug development, especially concerning cardiac medications and treatments that modulate muscle contraction.
Interplay in Muscle Contraction
Actin:
Actin primarily provides the thin filaments that serve as the scaffold for muscle contraction. Actin filaments have active sites where the myosin heads can attach.
Myosin:
Myosin is the dynamic force generator in muscle contraction. Myosin heads, powered by ATP hydrolysis, form cross-bridges with Actin and undergo power strokes, resulting in the sliding of Actin and Myosin filaments and muscle shortening.
In summary, Actin provides the structure, while Myosin generates the force necessary for muscle contraction, and the interplay between these proteins is at the heart of this crucial physiological process.
Implications in Biotechnology
Actin:
Actin is a fundamental protein used in various biotechnology applications, including:
- Microscopy: Actin is frequently labeled with fluorescent tags to visualize the cytoskeleton and cellular processes under the microscope.
- Cell Culture: Actin can be manipulated to study cell motility and migration, making it valuable in cell culture experiments.
Myosin:
Myosin and its molecular motors are harnessed in biotechnology for applications such as:
- In Vitro Motility Assays: Myosin-based motility assays are used to study the movement of molecular motors along Actin filaments, providing insights into motor protein function.
- Drug Screening: Myosin inhibitors or activators are screened in drug development for their impact on muscle contraction and associated diseases.
In summary, both Actin and Myosin have significant utility in biotechnology, with Actin being valuable for microscopy and cell culture, while Myosin is used in motility assays and drug screening.
Role in Aging
Actin:
Actin’s involvement in maintaining cell shape and integrity is linked to the aging process. Changes in Actin organization can contribute to age-related alterations in cell structure and function.
Myosin:
In the context of aging, Myosin has critical relevance in muscle health. Age-related declines in muscle mass and strength, known as sarcopenia, are associated with changes in Myosin function and muscle protein synthesis.
In summary, both Actin and Myosin play roles in the aging process, with Actin’s impact on cell structure and Myosin’s connection to muscle health in later years.
Clinical Applications
Actin:
Actin-based therapies are less common due to the challenge of selectively targeting Actin without affecting essential cellular processes. However, Actin’s involvement in cancer cell motility may hold promise for future therapeutic approaches.
Myosin:
Clinical applications related to Myosin primarily involve cardiac medications, such as myosin activators or myosin inhibitors, to treat heart conditions like heart failure.
In summary, while Actin-based therapies are less explored, Myosin-related clinical applications are gaining momentum in the treatment of heart-related disorders.
Beyond Muscle Contraction
Actin:
Actin’s role extends well beyond muscle contraction, as it is involved in a wide range of cellular functions. Its versatility in maintaining cell shape, aiding in cell motility, and contributing to intracellular processes makes it a multifunctional protein.
Myosin:
Myosin’s functions are not limited to muscle contraction. Its various isoforms play essential roles in cell motility, intracellular transport, and even non-muscle contractility, making it a diverse and versatile protein.
In summary, both Actin and Myosin have multifaceted roles that go beyond muscle contraction, encompassing an array of cellular processes that are crucial for life and health.
Involvement in Cardiac Health
Actin:
Actin has relevance in cardiac health through its role in muscle contraction in the heart. Genetic mutations affecting Actin can lead to various forms of cardiomyopathy, impacting heart function.
Myosin:
Myosin is intricately tied to cardiac health. Mutations in Myosin genes can cause hypertrophic cardiomyopathy, a condition characterized by the thickening of the heart muscle. Drugs targeting Myosin function are explored for the treatment of heart conditions.
In summary, both Actin and Myosin have implications for cardiac health, with Actin contributing to the structure of cardiac muscle, and Myosin affecting heart function and contractility.
Conclusion
Actin and Myosin are remarkable proteins with distinct but interdependent roles in the world of cellular biology. While Actin forms the structural backbone of the cell’s cytoskeleton and participates in a multitude of cellular processes, Myosin generates the mechanical force required for muscle contraction and various cellular movements. The intricate interplay between Actin and Myosin is at the core of muscle contraction and cell motility, shaping our ability to move and function.
These proteins are not limited to muscle cells; they play vital roles in non-muscle cells, biotechnology, aging, clinical applications, and cardiac health. Understanding the differences between Actin and Myosin is essential for unraveling the mysteries of life and the mechanisms behind various diseases.

FAQs
Actin and Myosin are two essential proteins found in cells, with distinct roles. Actin is a thin, globular protein that provides structural support to the cell and plays a role in various cellular processes, while Myosin is a thick, multi-chain protein responsible for generating mechanical force, particularly in muscle contraction.
Actin is ubiquitously distributed throughout the cell, with a strong presence in the cytoskeleton and various cellular structures. Myosin’s localization varies depending on the cell type and function, with a prominent role in muscle cells but also found in non-muscle cells and associated with organelles.
In muscle contraction, Actin and Myosin work together. Actin forms the thin filaments, providing the structure, and exposes binding sites for Myosin heads. Myosin forms the thick filaments, and its heads bind to Actin, undergo a power stroke powered by ATP hydrolysis, and generate the force needed for muscle contraction.
Actin does not directly consume energy during muscle contraction or cellular processes. Instead, the energy required for Actin’s functions is indirectly provided through ATP hydrolysis by Myosin. Myosin, in contrast, directly hydrolyzes ATP to generate mechanical force for muscle contraction and cell motility.
Yes, both Actin and Myosin are associated with various diseases. Mutations in Actin genes can lead to cardiomyopathies, cytoskeletal disorders, and their role in cell motility contributes to cancer metastasis. Myosin mutations are linked to myopathies, hypertrophic cardiomyopathy, neurological disorders, and also play a role in cancer metastasis.
Actin is highly conserved across diverse organisms, indicating its fundamental role in cellular processes. Myosin is also highly conserved but exhibits greater diversity with distinct isoforms for different cell types and functions.
While Actin is less commonly targeted in drug development due to its essential roles, Myosin is of growing interest, especially in the development of cardiac medications aimed at modulating muscle contraction and treating heart conditions.
Actin is involved in a wide range of cellular processes, including maintaining cell shape, cell motility, endocytosis, and exocytosis. Myosin has diverse functions beyond muscle contraction, participating in cell motility, intracellular transport, cytokinesis, and more.
Actin is associated with genetic cardiomyopathies that affect heart function. Myosin is linked to hypertrophic cardiomyopathy and is explored in the context of heart-related medications.
Actin’s role in maintaining cell structure has implications for aging-related changes in cell integrity. Myosin is relevant to age-related muscle changes, including age-related muscle mass and strength decline (sarcopenia).
Read More:
Contents
- Differences Between Actin and Myosin
- Structure and Composition
- Function
- Regulation of Activity
- Cellular Localization
- Energy Utilization
- Role in Muscle Contraction
- Involvement in Cell Motility
- Genetic Variants
- Interactions with Other Proteins
- Role in Diseases
- Evolutionary Aspects
- Role in Non-Muscle Cells
- Role in Muscle Types
- Significance in Drug Development
- Interplay in Muscle Contraction
- Implications in Biotechnology
- Role in Aging
- Clinical Applications
- Beyond Muscle Contraction
- Involvement in Cardiac Health
- Conclusion
- FAQs