| Property | Bromocresol Blue | Bromocresol Purple |
|---|---|---|
| Chemical Formula | C21H16Br4O5S | C21H16Br4O5S |
| Molecular Weight | Approximately 698.98 g/mol | Approximately 698.98 g/mol |
| Appearance | Blue powder or granules | Purple powder or granules |
| Solubility | Soluble in water | Soluble in water |
| pH Range of Sensitivity | 6.0 to 7.6 | 5.2 to 6.8 |
| Color in Alkaline (pH) | Blue (pH > 6.0) | Purple (pH > 6.8) |
| Color in Acidic (pH) | Yellow (pH < 6.0) | Yellow (pH < 5.2) |
| Equilibrium Constant (pKa) | Approximately 4.6 | Approximately 6.3 |
| Class of pH Indicator | Triphenylmethane dye | Thymol blue derivative |
| Specific Applications | Titration, electrophoresis, quality control | Serum albumin measurement, microbiology, water quality analysis |
| Solvent Compatibility | Water, ethanol, acetone, various organic solvents | Water, various organic solvents |
| Stability and Shelf Life | Stable under proper storage for several years | Stable under proper storage for an extended period |
| Toxicity and Safety | Generally safe when handled properly | Generally safe when handled properly |
| Cost Considerations | Cost-effective for routine testing | Reasonable cost, may be slightly higher |
| Environmental Impact | Considered safe for the environment when handled and disposed of responsibly | Considered safe for the environment when handled and disposed of responsibly |
Are you ready to become a pH maestro and make informed decisions in your lab experiments? Whether you’re a budding scientist or a seasoned researcher, understanding the nuances of Bromocresol Blue and Bromocresol Purple can be a game-changer. These pH indicators, with their mesmerizing color transformations, play a vital role in deciphering the acidity or alkalinity of solutions.
Differences Between Bromocresol Blue and Bromocresol Purple
The main differences between Bromocresol Blue and Bromocresol Purple lie in their pH sensitivity ranges and resulting color changes. Bromocresol Blue is most sensitive in the pH range of 6.0 to 7.6, transitioning from blue to yellow as the pH decreases, making it ideal for discerning slight pH changes in this range. In contrast, Bromocresol Purple is tailored to the pH range of 5.2 to 6.8, shifting from yellow to purple as the pH increases, making it more suitable for applications within this specific pH spectrum. These indicators are essential tools in laboratories, each excelling in distinct pH ranges, ensuring precise pH monitoring and accurate results in various scientific experiments and applications.
What Are Bromocresol Blue and Bromocresol Purple?
Bromocresol Blue and Bromocresol Purple are both synthetic pH indicators, meaning they change color in response to variations in the pH level of a solution. These indicators serve as invaluable tools for chemists, biologists, and researchers across various fields.
Bromocresol Blue:
Bromocresol Blue, often abbreviated as BCB, is a pH indicator that belongs to the triphenylmethane dye class. This compound is typically found as a powder or granules and is soluble in water. Bromocresol Blue is known for its vibrant blue color in an alkaline or basic environment (pH above 6.0) and its transition to a yellow color in an acidic environment (pH below 6.0). It exhibits a characteristic absorption maximum at approximately 602 nanometers when it appears blue and around 430 nanometers when it turns yellow.
| Property | Value |
|---|---|
| Chemical Formula | C21H16Br4O5S |
| Molecular Weight | Approximately 698.98 g/mol |
| Appearance | Blue powder or granules |
| Solubility | Soluble in water |
| Color in Alkaline (pH) | Blue (pH > 6.0) |
| Color in Acidic (pH) | Yellow (pH < 6.0) |
| Absorption Max (Blue) | ~602 nm |
| Absorption Max (Yellow) | ~430 nm |
Bromocresol Purple:
On the other hand, Bromocresol Purple, often abbreviated as BCP, is another pH indicator that belongs to the thymol blue class of pH indicators. Similar to Bromocresol Blue, Bromocresol Purple is available in the form of a powder or granules and is also soluble in water. Its distinguishing feature is its transition from yellow to purple in response to changes in pH, making it particularly useful in the pH range of 5.2 to 6.8.
| Property | Value |
|---|---|
| Chemical Formula | C21H16Br4O5S |
| Molecular Weight | Approximately 698.98 g/mol |
| Appearance | Purple powder or granules |
| Solubility | Soluble in water |
| Color in Alkaline (pH) | Purple (pH > 6.8) |
| Color in Acidic (pH) | Yellow (pH < 5.2) |
| Transition pH Range | 5.2 to 6.8 |
The pH Range of Sensitivity
One of the primary differences between Bromocresol Blue and Bromocresol Purple lies in their pH sensitivity ranges.
Bromocresol Blue:
Bromocresol Blue is most sensitive in the pH range of 6.0 to 7.6, which makes it an ideal choice for detecting slight changes in the pH of solutions in this range. Its vivid transition from blue to yellow within this pH window allows scientists to pinpoint whether a solution is slightly alkaline or slightly acidic.
Bromocresol Purple:
Conversely, Bromocresol Purple is tailored to the pH range of 5.2 to 6.8. This indicator is especially useful for distinguishing between mildly acidic and mildly alkaline solutions. When the pH falls below 5.2, it turns yellow, and when the pH rises above 6.8, it adopts a purple hue.
Applications in Laboratories
Both Bromocresol Blue and Bromocresol Purple have a wide range of applications in laboratory settings due to their distinct pH response characteristics.
Bromocresol Blue Applications:
- Titration: Bromocresol Blue is commonly used in acid-base titrations, where it serves as a visual indicator of the endpoint of the titration process. When the solution reaches a neutral pH, Bromocresol Blue undergoes its characteristic color change from blue to yellow, signaling the completion of the reaction.
- Electrophoresis: In gel electrophoresis, a technique used to separate molecules like DNA or proteins, Bromocresol Blue is added to the loading buffer to monitor the progress of electrophoresis. It helps researchers track the movement of molecules through the gel, as the color change indicates their migration.
- Quality Control: Bromocresol Blue is also used in quality control processes for assessing the pH of various products, such as beverages and pharmaceuticals. Its sensitivity in the pH range of 6.0 to 7.6 makes it suitable for detecting slight deviations from the desired pH.
Bromocresol Purple Applications:
- Serum Albumin Measurement: Bromocresol Purple finds its application in clinical laboratories to measure serum albumin levels in blood. The color change of Bromocresol Purple is directly proportional to the concentration of albumin, allowing for precise quantification.
- pH Indicator in Microbiology: In microbiology, Bromocresol Purple is used as a pH indicator in growth media for bacteria. It helps microbiologists differentiate between bacteria that can ferment carbohydrates and those that cannot, based on the pH changes resulting from fermentation.
- Detecting Ammonium Nitrogen: Bromocresol Purple is employed in environmental testing to detect ammonium nitrogen in water samples. The color change from purple to yellow indicates the presence of ammonium ions, providing vital information about water quality.
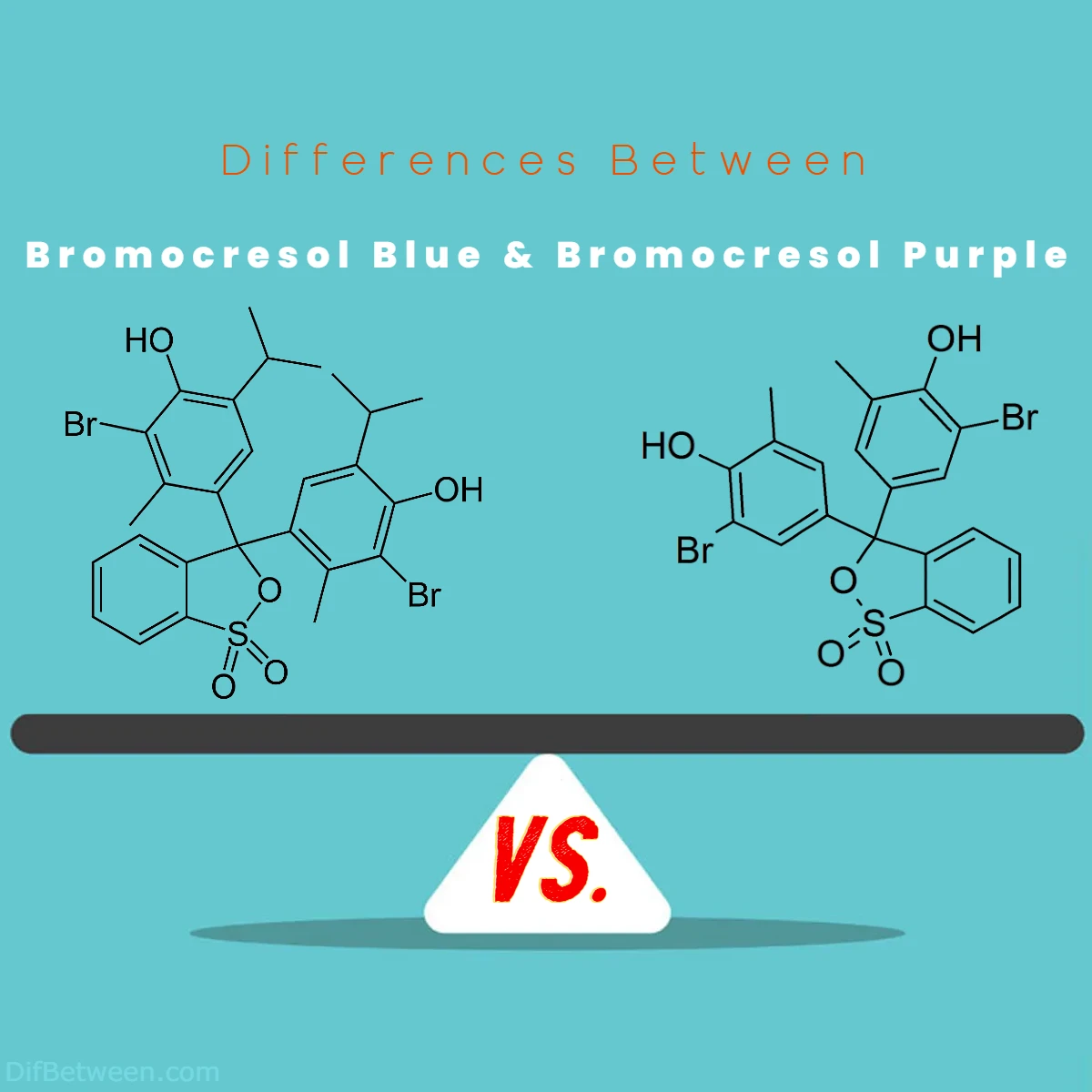
Chemical Structures and Composition
While both Bromocresol Blue and Bromocresol Purple share similar chemical formulas and molecular weights, they belong to different classes of pH indicators, leading to subtle differences in their chemical structures.
Bromocresol Blue Structure:
Bromocresol Blue has a chemical formula of C21H16Br4O5S and belongs to the triphenylmethane dye class. It contains multiple aromatic rings and bromine atoms, which are responsible for its color-changing properties. The structural arrangement of atoms in Bromocresol Blue results in its characteristic blue and yellow colors at different pH levels.
Bromocresol Purple Structure:
Similarly, Bromocresol Purple also has the chemical formula C21H16Br4O5S but belongs to the thymol blue class of pH indicators. Its chemical structure includes aromatic rings and bromine atoms, which give rise to its yellow and purple colors as pH changes. However, the specific arrangement of these atoms differs from Bromocresol Blue, accounting for the distinct pH range of sensitivity.
Comparing Color Changes
The most striking difference between Bromocresol Blue and Bromocresol Purple is, of course, their color changes in response to pH variations.
Bromocresol Blue Color Changes:
Bromocresol Blue is renowned for its vivid transition from blue to yellow. Below a pH of 6.0, it appears yellow, while above a pH of 6.0, it adopts a vibrant blue hue. This stark contrast in colors makes it easy for researchers to visually determine whether a solution is acidic or alkaline.
Bromocresol Purple Color Changes:
In contrast, Bromocresol Purple undergoes a transition from yellow to purple. When the pH falls below 5.2, it turns yellow, and when the pH rises above 6.8, it shifts to a purple color. This unique color change pattern is particularly useful for detecting pH variations within the mildly acidic to mildly alkaline range.
Compatibility with Different Substances
Another crucial aspect to consider when choosing between Bromocresol Blue and Bromocresol Purple is their compatibility with various substances and solvents.
Bromocresol Blue Compatibility:
Bromocresol Blue is generally compatible with a wide range of solvents, including water, ethanol, and acetone. This versatility makes it suitable for use in aqueous solutions as well as organic solvents, expanding its applicability in different laboratory procedures.
Bromocresol Purple Compatibility:
Similarly, Bromocresol Purple is soluble in water and various organic solvents. Its compatibility with different solvents allows for its use in diverse applications, such as clinical assays and environmental testing.
Stability and Shelf Life
When it comes to the stability and shelf life of these pH indicators, there are some noteworthy differences to consider.
Bromocresol Blue Stability:
Bromocresol Blue is known for its relatively stable nature when stored in dry conditions away from light and air. When handled properly, it can have a shelf life of several years without significant degradation in its color-changing properties.
Bromocresol Purple Stability:
Bromocresol Purple also exhibits good stability, but it may be slightly less stable than Bromocresol Blue in certain storage conditions. It is still essential to store Bromocresol Purple in a cool, dry place away from light and air to maximize its shelf life.
Toxicity and Safety
The safety of laboratory chemicals is always a top priority, and both Bromocresol Blue and Bromocresol Purple have safety considerations to keep in mind.
Bromocresol Blue Safety:
Bromocresol Blue is generally considered safe to handle when used according to standard laboratory practices. It is not known to be highly toxic, but like many chemicals, it should be handled with care to avoid ingestion, inhalation, or contact with skin and eyes.
Bromocresol Purple Safety:
Similarly, Bromocresol Purple is regarded as safe for laboratory use when handled responsibly. While it is not highly toxic, it is essential to follow safety guidelines and wear appropriate protective gear when working with this indicator to prevent any potential risks.
Cost Considerations
Cost can often be a determining factor when selecting laboratory reagents. Let’s compare the cost-effectiveness of Bromocresol Blue and Bromocresol Purple.
Bromocresol Blue Cost:
Bromocresol Blue is typically more widely available and may be more cost-effective than Bromocresol Purple. Its affordability makes it a preferred choice for routine laboratory testing where precise pH measurement within its sensitivity range is not critical.
Bromocresol Purple Cost:
Bromocresol Purple, while still reasonably priced, may be slightly more expensive than Bromocresol Blue due to factors such as production processes and market demand. However, its unique pH range sensitivity justifies its cost in applications where it is specifically required.
Equilibrium Constants (pKa)
The equilibrium constant, often denoted as pKa, is a measure of the strength of an acid or base. It plays a significant role in understanding the behavior of pH indicators.
Bromocresol Blue pKa:
The pKa of Bromocresol Blue is approximately 4.6. This value indicates that Bromocresol Blue exists primarily in its acidic form below pH 4.6 and in its basic form above pH 4.6. The midpoint of its color transition aligns with this pKa value, making it highly responsive to pH changes near this point.
Bromocresol Purple pKa:
Bromocresol Purple has a pKa value of approximately 6.3. This means that it predominantly exists as an acidic form below pH 6.3 and as a basic form above pH 6.3. Its color transition is centered around this pKa, ensuring optimal sensitivity within the pH range it is designed for.
Compatibility with Solvents
Both Bromocresol Blue and Bromocresol Purple are prized for their compatibility with various solvents, broadening their utility.
Bromocresol Blue Solvent Compatibility:
Bromocresol Blue exhibits excellent solubility in water, making it an ideal choice for aqueous solutions. Additionally, it is soluble in organic solvents like ethanol and acetone, expanding its versatility for different laboratory applications.
Bromocresol Purple Solvent Compatibility:
Similar to Bromocresol Blue, Bromocresol Purple is soluble in water, rendering it suitable for aqueous solutions. It also demonstrates compatibility with organic solvents, ensuring its adaptability to various experimental setups.
Environmental Impact
In today’s environmentally conscious world, it’s crucial to consider the environmental impact of chemicals used in laboratories.
Bromocresol Blue Environmental Impact:
Bromocresol Blue is considered safe for the environment when used responsibly and disposed of properly. It is not classified as hazardous waste, and its use in laboratories is generally in compliance with environmental regulations.
Bromocresol Purple Environmental Impact:
Likewise, Bromocresol Purple is considered safe for the environment when handled and disposed of responsibly. Proper disposal methods should be followed to minimize any potential impact on the ecosystem.
Bromocresol Blue or Bromocresol Purple: Which One is Right Choose for You?
Choosing between Bromocresol Blue and Bromocresol Purple can be a critical decision in your laboratory work, as it directly impacts the accuracy and effectiveness of your experiments. To help you make the right choice, let’s simplify the decision-making process by considering specific scenarios and needs.
When to Choose Bromocresol Blue:
- pH Range – Slightly Alkaline to Slightly Acidic: If your experiments primarily involve solutions with a pH range of 6.0 to 7.6 or require detecting slight pH changes within this range, Bromocresol Blue is the ideal choice. Its sharp blue-to-yellow transition in this pH window ensures precise pH monitoring.
- Cost-Effectiveness: If you’re working on routine laboratory testing where precise pH measurement within its sensitivity range is sufficient, Bromocresol Blue is often more cost-effective and readily available.
- Versatile Solvent Compatibility: Bromocresol Blue is compatible with a wide range of solvents, including water, ethanol, and acetone, making it suitable for various experimental setups.
- Stability and Shelf Life: When stored correctly, Bromocresol Blue maintains its color-changing properties for several years, ensuring long-term usability.
- Safety: Bromocresol Blue is generally safe when handled following standard laboratory safety practices.
When to Choose Bromocresol Purple:
- pH Range – Mildly Acidic to Mildly Alkaline: If your experiments involve solutions within the pH range of 5.2 to 6.8 or require precise differentiation within this range, Bromocresol Purple is the preferred option. Its unique yellow-to-purple shift is specifically tailored to this pH window.
- Clinical and Biological Applications: In clinical laboratories or microbiology, where you need to measure serum albumin levels, differentiate between bacteria based on pH changes, or detect ammonium ions in water samples, Bromocresol Purple excels.
- Environmental Testing: For environmental testing applications, such as water quality analysis where the presence of ammonium nitrogen needs to be assessed, Bromocresol Purple is a valuable choice.
- Solvent Compatibility: Similar to Bromocresol Blue, Bromocresol Purple is compatible with water and various organic solvents, ensuring adaptability to different experimental requirements.
- Safety: When handled with proper safety precautions, Bromocresol Purple is considered safe for laboratory use.
- Specific pH Requirements: If your experiments demand pH monitoring within the Bromocresol Purple’s sensitivity range, choosing it ensures accurate and reliable results.
Conclusion:
In the end, the choice between Bromocresol Blue and Bromocresol Purple boils down to the pH range of your experiments and specific application requirements. Bromocresol Blue is ideal for solutions in the pH range of 6.0 to 7.6 and cost-effective routine testing. On the other hand, Bromocresol Purple excels in the pH range of 5.2 to 6.8, making it indispensable for clinical, biological, and environmental applications where precision within this range is crucial.
Consider your experiment’s pH range, sensitivity needs, and intended applications to make the best choice between these two valuable pH indicators.
FAQs
Bromocresol Blue and Bromocresol Purple are synthetic pH indicators used in chemistry and biology to determine the acidity or alkalinity of solutions. They change color in response to pH variations.
Bromocresol Blue is most sensitive in the pH range of 6.0 to 7.6. It transitions from blue to yellow as the pH decreases.
Bromocresol Purple is sensitive in the pH range of 5.2 to 6.8. It shifts from yellow to purple as the pH increases.
Choose Bromocresol Blue for pH monitoring in the range of 6.0 to 7.6, ideal for slight pH changes and routine testing. Opt for Bromocresol Purple for pH within the range of 5.2 to 6.8, suitable for clinical, biological, or environmental applications requiring precision in that range.
Yes, both indicators are generally considered safe when handled following standard laboratory safety practices.
Both indicators are soluble in water and various organic solvents, making them adaptable to different experimental setups.
It is best to choose the indicator that aligns with your desired pH range and sensitivity requirements, as they are optimized for different pH spectrums.
When stored properly in dry, cool conditions away from light and air, both indicators can maintain their color-changing properties for an extended period, typically several years.
When handled and disposed of responsibly, both Bromocresol Blue and Bromocresol Purple are considered safe for the environment.
Bromocresol Blue is often used in acid-base titrations, electrophoresis, and quality control processes. Bromocresol Purple is employed in serum albumin measurement, microbiology, and water quality analysis, among other applications.
Read More:
Contents
- Differences Between Bromocresol Blue and Bromocresol Purple
- What Are Bromocresol Blue and Bromocresol Purple?
- The pH Range of Sensitivity
- Applications in Laboratories
- Chemical Structures and Composition
- Comparing Color Changes
- Compatibility with Different Substances
- Stability and Shelf Life
- Toxicity and Safety
- Cost Considerations
- Equilibrium Constants (pKa)
- Compatibility with Solvents
- Environmental Impact
- Bromocresol Blue or Bromocresol Purple: Which One is Right Choose for You?
- FAQs