| Aspect | Dilution | Titration |
|---|---|---|
| Purpose | Reduce concentration, adjust solutions | Determine unknown concentration |
| Process | Mixing a solution with a solvent | Controlled chemical reaction |
| Equipment | Basic glassware (beakers, flasks, etc.) | Burette, indicator, specialized glassware |
| Chemical Reactions | No chemical reactions involved | Involves chemical reactions |
| Endpoint Detection | No specific endpoint | Endpoint marked by color change |
| Calculation | Based on dilution equation | Stoichiometric calculations |
| Applications | – Pharmaceutical formulations – Biological research – Chemical analysis | – Acid-base titration – Redox titration – Pharmaceutical quality control |
| Precision and Accuracy | Precise with careful measurement | Highly accurate with proper technique |
| Sensitivity to Errors | Errors due to volume measurement | Errors in equipment calibration, mixing |
| Complexity of Calculations | Simple dilution equation | Stoichiometric calculations |
| Safety Considerations | Generally safe | May involve hazardous chemicals |
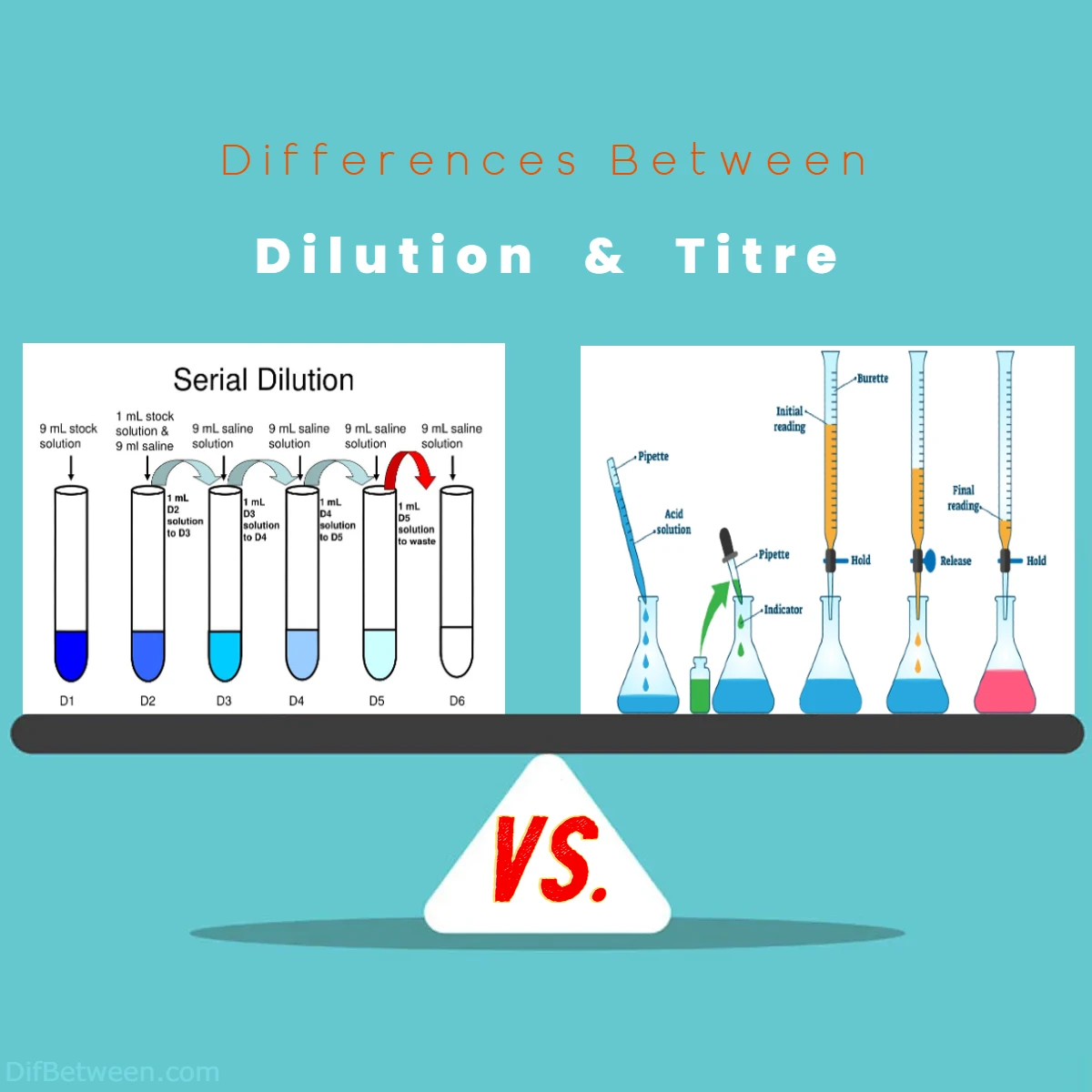
Dilution, like a skillful painter’s gentle strokes on a canvas, allows us to reduce the intensity of a solution while retaining its essence. It’s the art of adding just the right touch of solvent to create a masterpiece of desired concentration. On the other hand, titre, akin to a detective solving a mystery, helps us determine the concentration of unknown solutions. It’s the process of carefully mixing known and unknown substances until they reveal their secrets through a dramatic change in color.
Differences Between Dilution and Titre
The main differences between Dilution and Titre lie in their respective purposes and processes. Dilution involves reducing the concentration of a solution by adding a solvent, often to make it safer, more manageable, or cost-effective. In contrast, Titre is employed to precisely determine the concentration of a solution, particularly when it’s unknown, through a controlled chemical reaction, typically signaled by a distinct change in color. While dilution relies on mathematical calculations to achieve desired concentrations, titration demands specialized equipment, such as a burette and an indicator, to reach a precise endpoint, making it a fundamental technique in analytical chemistry and quality control.
1. Purpose:
- Dilution: Dilution is primarily used to reduce the concentration of a solution, either for safety, precision, or cost-effectiveness. The goal is to create a solution with a desired concentration.
- Titration: Titration, on the other hand, is used to determine the precise concentration of a solution of unknown concentration. It involves the reaction of the unknown solution with a solution of known concentration until a chemical reaction reaches completion, signified by an indicator.
2. Process:
- Dilution: The process of dilution involves straightforward mathematical calculations based on the dilution equation. It doesn’t typically involve chemical reactions.
- Titration: Titration is a dynamic process that requires careful addition of a titrant to an analyte. It involves a chemical reaction that eventually reaches an endpoint indicated by a color change.
3. Equipment:
- Dilution: Dilution usually requires basic laboratory glassware like beakers, flasks, and pipettes. It’s a relatively simple process in terms of equipment.
- Titration: Titration involves more specialized equipment, including a burette for precise volume measurement and an indicator to signal the endpoint. It also requires a titration flask or vessel.
4. Chemical Reactions:
- Dilution: Dilution does not involve chemical reactions. It is a physical process that involves mixing a solution with a solvent.
- Titration: Titration is fundamentally based on a chemical reaction between the analyte and titrant. The reaction is carefully monitored to determine the endpoint.
5. Endpoint Determination:
- Dilution: Dilution does not have an endpoint in the same sense as titration. The endpoint of dilution is simply the point at which you have added the desired amount of solvent.
- Titration: Titration has a well-defined endpoint signaled by a noticeable change, such as a color change in the indicator. This is a critical aspect of titration, as it marks the completion of the reaction.
6. Calculation:
- Dilution: The calculation in dilution is relatively straightforward and is based on the dilution equation. It involves determining the volume of stock solution and solvent needed to achieve the desired concentration.
- Titration: Titration calculations are more complex as they involve stoichiometry and require knowledge of the balanced chemical equation for the reaction between the analyte and titrant. The concentration of the analyte is calculated based on the volume and concentration of the titrant used.
7. Practical Applications
Dilution Applications:
1. Pharmaceutical Industry
In pharmaceutical manufacturing, precise drug concentrations are vital. Dilution is commonly employed to prepare drug formulations with the required therapeutic potency. Pharmaceutical companies often use dilution to adjust the concentration of active pharmaceutical ingredients (APIs) to meet regulatory standards.
2. Chemical Analysis
Analytical chemistry relies heavily on dilution to work with samples of known or unknown concentrations. For instance, when conducting flame atomic absorption spectroscopy (FAAS) or inductively coupled plasma mass spectrometry (ICP-MS), samples are often diluted to a manageable concentration range for accurate measurements.
3. Biological Research
Biological experiments often require working with solutions of various concentrations. Dilution is frequently used to prepare culture media, reagents, and buffer solutions in microbiology, cell biology, and molecular biology laboratories.
Titration Applications:
1. Acid-Base Titration
One of the most common applications of titration is in determining the concentration of acids or bases. This is crucial in analytical chemistry, where titration is used to measure the acidity or alkalinity of solutions, determine the purity of chemicals, and ensure product quality control.
2. Redox Titration
Titration is employed in redox reactions to determine the concentration of oxidizing or reducing agents. For example, in environmental chemistry, titration can be used to assess the concentration of pollutants, such as the level of dissolved oxygen in water.
3. Pharmaceutical Quality Control
Pharmaceutical companies use titration techniques to verify the purity and concentration of drug formulations. This ensures that medications meet stringent quality standards and are safe and effective for patients.
8. Precision and Accuracy
Dilution:
Dilution is a precise process when performed correctly. The dilution equation allows for accurate control of the final concentration. However, errors can occur due to issues like inaccurate measurement of volumes or miscalculation. Proper pipetting and careful measurement are crucial to achieve precision.
Titration:
Titration is renowned for its accuracy, particularly when performed using standardized solutions and meticulous techniques. The endpoint, marked by a distinct color change, is a clear indicator of when the reaction is complete. This precision makes titration invaluable for determining unknown concentrations.
9. Equipment and Chemicals
Dilution:
Dilution typically requires minimal specialized equipment, making it accessible for routine laboratory tasks. Common glassware, such as pipettes, beakers, and volumetric flasks, suffices. The primary chemicals involved are the stock solution and the diluent (usually water).
Titration:
Titration demands specific equipment, including a burette for precise volume delivery, an indicator to signal the endpoint, and a titration flask or vessel. Additionally, you need standardized solutions of known concentration for accurate titration. The choice of indicator depends on the specific reaction being studied.
10. Sensitivity to Errors
Dilution:
Errors in dilution often stem from imprecise measurement of volumes or inaccuracies in the concentration of the stock solution. While these errors can affect the final concentration, they are usually straightforward to identify and correct.
Titration:
Titration is sensitive to various factors that can introduce errors. These include errors in the calibration of equipment, the choice of indicator, and incomplete mixing of reactants. Careful technique and calibration are critical to minimizing errors in titration.
11. Complexity of Calculations
Dilution:
Dilution calculations are relatively straightforward and are based on the dilution equation (C1V1 = C2V2). Once you have the initial and desired concentrations, as well as the volumes involved, the calculation is simple and direct.
Titration:
Titration calculations can be more complex, especially in cases where the reaction involves multiple steps or the stoichiometry is not 1:1. Stoichiometric calculations are essential to determine the analyte’s concentration accurately based on the volume and concentration of the titrant used.
12. Safety Considerations
Dilution:
Dilution is generally a safe process, especially when dealing with non-reactive substances. However, when diluting hazardous chemicals, proper safety measures must be in place to minimize exposure and potential risks.
Titration:
Titration can involve reactive substances and chemical reactions. Safety precautions, such as the use of appropriate personal protective equipment (PPE) and proper ventilation, are essential to ensure the safety of personnel.
Summary
In summary, both dilution and titration are essential techniques in the laboratory, each with its unique purpose, applications, and characteristics. Dilution is primarily used to adjust concentrations for various reasons, while titration is employed to precisely determine the concentration of a solution. Dilution is a relatively straightforward process with minimal specialized equipment, while titration demands specific apparatus and standardized solutions.
Understanding the differences between these techniques is crucial for scientists and researchers to choose the appropriate method for their specific needs. Whether you’re preparing solutions with specific concentrations through dilution or accurately quantifying unknown concentrations via titration, these techniques are indispensable tools in scientific exploration and analysis.
FAQs
Dilution is the process of reducing the concentration of a solution by adding a solvent. It is used to adjust concentrations for various reasons, such as safety, precision, or cost-effectiveness.
Titration is a laboratory technique used to determine the precise concentration of a solution, especially when it’s unknown. Its primary purpose is quantitative analysis and quality control.
Dilution involves mixing a solution with a solvent to achieve a desired concentration without a chemical reaction. In contrast, titration involves a controlled chemical reaction between the analyte and a titrant to determine concentration.
Dilution commonly requires basic laboratory glassware like beakers, flasks, and pipettes. It’s a relatively simple process in terms of equipment.
Titration demands specific equipment, including a burette for precise volume measurement, an indicator to signal the endpoint, and a titration flask or vessel. Additionally, standardized solutions are required.
Dilution does not have a specific endpoint like titration. The endpoint in dilution is simply the point at which you have added the desired amount of solvent.
The endpoint of titration is detected by a noticeable change, typically a color change, in the indicator. This change indicates that the reaction between the analyte and titrant is complete.
Titration is generally more precise due to the accurate endpoint detection and rigorous calibration of equipment. Dilution can be precise when executed carefully but is inherently simpler.
Dilution is generally safe, especially when dealing with non-reactive substances. Titration, however, can involve hazardous chemicals and chemical reactions, necessitating safety precautions.
Dilution is used in various fields, including pharmaceuticals, chemical analysis, and biological research. Titration finds applications in acid-base titration, redox titration, and pharmaceutical quality control, among others.
Read More: