| Aspect | Polymers | Macromolecules |
|---|---|---|
| Definition | Large molecules composed of repeating monomers linked together in a chain-like fashion. | Massive molecules, often composed of thousands of atoms, exhibiting diverse structures. |
| Structural Unit | Monomers linked in linear or branched chains. | Complex three-dimensional structures with various types, including polymers. |
| Examples | Plastics, rubber, synthetic fibers. | Proteins, nucleic acids (DNA, RNA), polysaccharides. |
| Bonding | Primarily covalent bonds between monomers. | Covalent and non-covalent bonds shaping intricate structures. |
| Origin | Can be natural (cellulose, proteins) or synthetic (polyethylene, nylon). | Can be natural (proteins, nucleic acids) or synthetic (artificial proteins, synthetic DNA). |
| Properties | Diverse properties based on chain length, branching, and intermolecular forces. | Properties determined by intricate folding patterns, hydrogen bonds, and interactions within the molecule. |
| Applications | Packaging, textiles, medical implants. | Enzymes, drug delivery, genetic engineering, medical diagnostics. |
| Quantum Influence | Quantum effects impact properties and behavior at the molecular level. | Quantum mechanics influences bonding forces, structure, and functions. |
| Intermolecular Forces | Dictate polymer chain behavior and interactions. | Shape macromolecular folding patterns and functions. |
| Nanotechnology Role | Used in nanocomposites for enhanced properties. | Engineered for nanoscale functions, such as drug delivery and nanostructures. |
| Natural vs. Synthetic | Can be both natural (rubber) and synthetic (plastics). | Found in both natural (proteins) and synthetic (artificial proteins) forms. |
| Functionality | Properties often tailored for specific applications. | Intricate folding patterns determine biological functions. |
| Influence on Industries | Reshaped industries from packaging to aerospace. | Revolutionized medicine, biotechnology, and materials science. |
| Future Innovations | Continuing to explore new materials and applications. | Expanding into nanotechnology, advancing medical treatments, and beyond. |
Today, we’re diving into a captivating comparison between two fascinating entities: polymers and macromolecules. While these terms might sound somewhat similar, they each hold distinctive qualities that set them apart. So, buckle up as we explore the semantic nuances and entity marvels of polymer vs. macromolecule.
Differences Between Polymer and Macromolecule
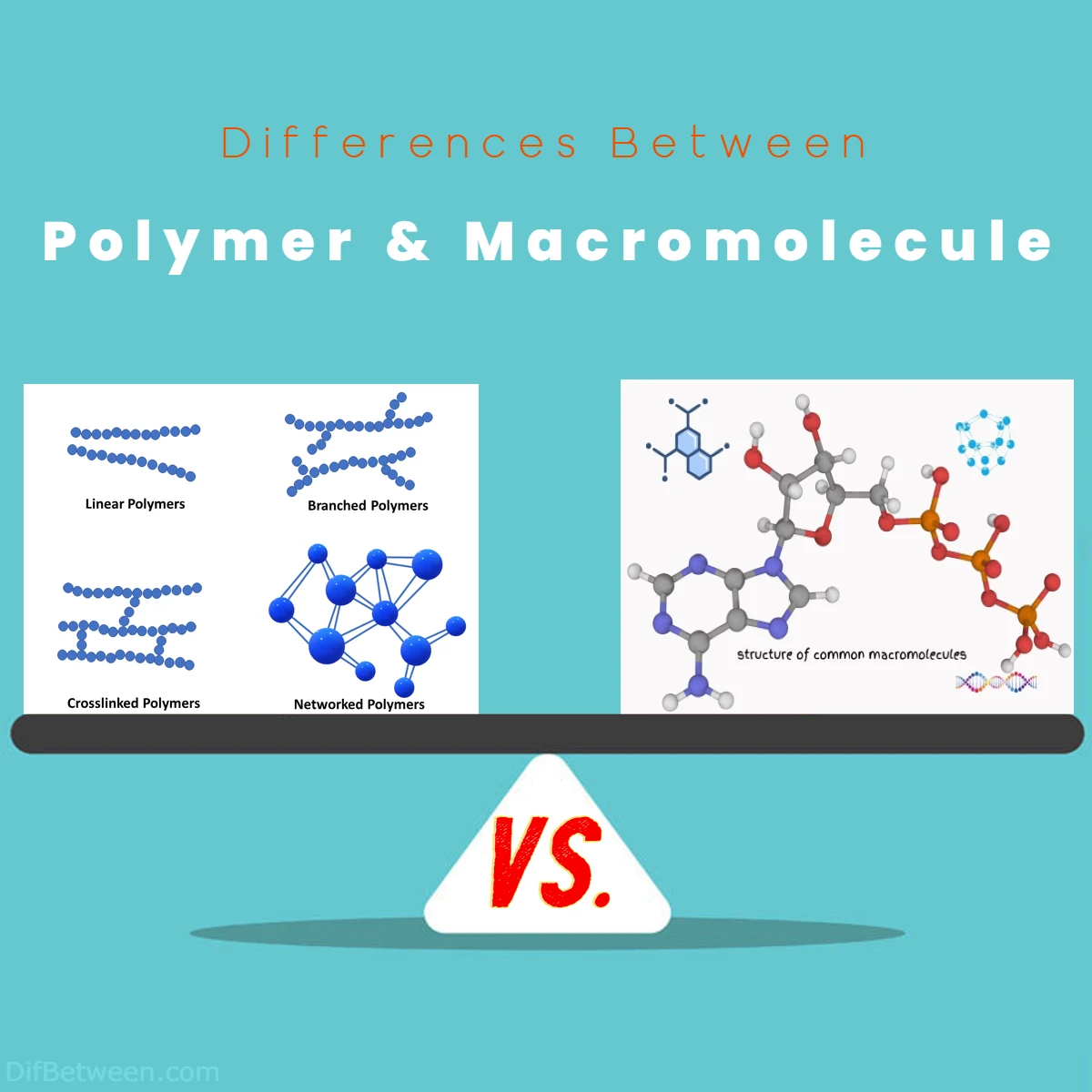
Polymers and macromolecules exhibit distinct structural and functional disparities in the world of chemistry. Polymers are sizable molecules formed by linking repeating monomers, often manifesting as linear or branched chains. In contrast, macromolecules, including proteins and nucleic acids like DNA, are colossal entities composed of thousands of atoms and are characterized by intricate three-dimensional structures. While polymers primarily rely on covalent bonds between monomers, macromolecules involve complex interactions, including covalent and non-covalent bonds, shaping their unique functions. These differences extend to their applications, where polymers find use in textiles, packaging, and medical devices, while macromolecules play pivotal roles in biology, genetics, and medicine.
Defining Polymers and Macromolecules
Let’s begin our adventure by understanding the basic essence of what polymers and macromolecules truly are.
Polymers: Building Blocks of Variety
Picture polymers as the architects of diversity in the molecular realm. These are large molecules composed of repeating structural units, called monomers, linked together in a chain-like fashion. The magic happens through a process called polymerization, where monomers join hands, creating a long chain with a distinct sequence.
In simpler terms, think of polymers as a string of beads, where each bead represents a monomer, and the string itself symbolizes the polymer chain. These chains can be long or short, simple or complex, giving rise to an astonishing array of materials with diverse properties. From the rubber in your sneakers to the plastics shaping our modern world, polymers are the silent heroes enhancing our daily lives.
Macromolecules: Beyond the Grande
Now, shift your focus to macromolecules, the colossal entities that make polymers possible. A macromolecule is essentially a massive molecule, often composed of several thousands of atoms. These molecules are no less than the giants of the molecular world, encompassing various types, including polymers.
Intriguingly, macromolecules can be composed of smaller molecules that aren’t monomers. Unlike polymers, they might not be repeating units; rather, they are characterized by their sheer size and complex structure. Macromolecules could be proteins, nucleic acids like DNA and RNA, or even certain natural and synthetic polymers. They’re the mammoths of the microscopic universe, showcasing the marvel of nature’s intricate designs.
Structural Distinctions: Polymer vs. Macromolecule
Now that we’ve laid the foundation, let’s delve into the structural disparities that set these entities apart.
Polymer Structure: The Chain Game
Polymers exhibit a specific arrangement that distinguishes them from other molecules. As we mentioned earlier, they consist of monomers linked together in a linear or branched manner. This linkage forms a chain, akin to a necklace made of identical beads.
Polymer chains can vary in length, and their sequential arrangement of monomers imparts unique characteristics to the material. This structural diversity is what makes different types of plastics, rubbers, and fibers possible. Take polyethylene, for instance, with its straightforward linear chain, and contrast it with the complexity of DNA, which is also a polymer but adopts a double-helix structure.
Macromolecule Structure: Heterogeneous Giants
Macromolecules, on the other hand, are a heterogeneous bunch when it comes to structure. They can encompass a range of molecular architectures, often far more intricate than the linear or branched chains seen in most polymers. Macromolecules include proteins, nucleic acids, and polysaccharides, each with a distinct three-dimensional arrangement.
Consider proteins: these macromolecules are built from a set of 20 amino acids, and their intricate folding patterns dictate their functions. The primary, secondary, tertiary, and quaternary structures of proteins are a testament to the complexity macromolecules can attain.
Natural vs. Synthetic: Origins and Production
Prepare to witness the clash of origins as we explore the natural and synthetic aspects of polymers and macromolecules.
Natural Polymers: Nature’s Artistry
Natural polymers are derived from living organisms and are a masterpiece of nature’s ingenuity. They’ve been around for eons, serving as essential building blocks in various biological processes. These polymers are often biodegradable, playing well with our environment. Cellulose, found in plant cell walls, and chitin, forming the exoskeletons of insects, are prime examples of natural polymers.
Synthetic Polymers: Human-Crafted Wonders
Step into the realm of human creativity, where synthetic polymers reign supreme. These polymers are meticulously engineered in laboratories and industrial settings. The beauty lies in their versatility—scientists can tailor their properties to suit specific applications. From the elasticity of synthetic rubber to the durability of nylon, these polymers have reshaped industries and everyday life.
Natural Macromolecules: The Essence of Life
Macromolecules in their natural form are the embodiment of life’s blueprint. Nucleic acids, carrying genetic information, and proteins, orchestrating biological functions, are prime examples. The intricate dance of DNA’s double helix and the intricate folds of proteins determine the grand symphony of life itself.
Synthetic Macromolecules: Bridges to Innovation
While nature’s macromolecules are awe-inspiring, scientists have ventured into creating their synthetic counterparts. Synthetic macromolecules often mimic the functions of their natural counterparts and can be tailored for various applications. Synthetic DNA, for instance, is used in genetic engineering, and artificial proteins are designed for medical and industrial purposes.
Properties and Applications: Where the Magic Happens
Get ready to uncover the real-world enchantment as we delve into the properties and applications that distinguish these entities.
Polymer Properties and Applications
The properties of polymers are as diverse as the colors of a rainbow. They can be flexible like rubber, sturdy like plastic, or even conductive like certain advanced materials. These properties stem from factors such as molecular weight, branching, and intermolecular forces.
Polymers find their way into nearly every aspect of our lives. You’ll encounter them in the bottles holding your drinks, the fabrics you wear, and the casings of electronic devices. The world of medicine also relies on polymers for drug delivery systems, medical implants, and biodegradable sutures.
Macromolecule Properties and Applications
Macromolecules, being the essential players in biological processes, boast properties that are a testament to the complexity of life itself. Proteins exhibit specificity in their binding, enabling enzymes to catalyze reactions with precision. Nucleic acids store and transmit genetic information, laying the foundation for inheritance.
In medicine, macromolecules are stars. Enzyme replacement therapies, utilizing proteins, are saving lives. Genetic engineering and gene therapy, centered around manipulating nucleic acids, hold the promise of treating genetic disorders.
Properties and Applications: Where Science Meets Reality
Prepare to be amazed as we unravel the remarkable properties and far-reaching applications that make polymers and macromolecules indispensable in our lives.
Polymer Properties and Applications
The world of polymers is a treasure trove of diversity, offering an array of properties tailor-made for various applications. From the stretchiness of elastomers to the rigidity of thermosetting plastics, polymers are versatile materials that adapt to our needs. Their lightweight nature, combined with durability, makes them essential in industries ranging from packaging to aerospace.
Macromolecule Properties and Applications
Macromolecules, the architects of life, boast properties that span a spectrum of functions. Proteins’ specific folding patterns allow them to be enzymes, catalysts that drive biochemical reactions. Nucleic acids’ ability to store and transmit genetic information paves the way for understanding and manipulating life’s processes. These properties find applications in medicine, research, and beyond.
Polymer or Macromolecule: Which One is Right Choose?
The choice between polymers and macromolecules depends on the context, application, and specific goals you have in mind. Both polymers and macromolecules are essential components of the molecular world, each with its unique attributes and applications. Let’s delve deeper into the considerations that might guide your choice:
Choosing Polymers:
- Versatility: If you’re looking for materials with a wide range of applications, polymers might be the way to go. Polymers can be engineered to exhibit diverse properties, from flexibility and elasticity to strength and thermal resistance.
- Industrial Impact: Polymers have reshaped industries, from packaging and textiles to electronics and medicine. If you’re interested in contributing to the development of new materials or improving existing ones, polymers offer a vast playground for innovation.
- Practical Applications: If you’re interested in creating everyday products or addressing practical challenges, polymers can be the answer. They’re used in everything from consumer goods to medical devices.
Choosing Macromolecules:
- Biological Significance: If you’re intrigued by the intricacies of life and biology, macromolecules like proteins and nucleic acids might be your focus. Understanding macromolecular structures and functions is fundamental to fields like medicine and genetics.
- Precision and Specificity: Macromolecules often exhibit precise functions and behaviors dictated by their complex structures. If you’re interested in designing molecules for targeted applications, such as drug delivery or genetic engineering, macromolecules provide a platform for this level of precision.
- Cutting-Edge Fields: If you’re drawn to emerging fields like nanotechnology or synthetic biology, macromolecules play a pivotal role. Their manipulation at the molecular level is central to these groundbreaking areas of research and innovation.
Ultimately, the choice between polymers and macromolecules depends on your interests, the scientific questions you want to explore, and the impact you wish to make. It’s worth noting that polymers and macromolecules are not mutually exclusive; rather, they complement each other in various contexts. Many synthetic polymers are also macromolecules, and understanding the interactions between these entities can lead to novel discoveries.
In the grand tapestry of science, both polymers and macromolecules are threads that contribute to our understanding of the molecular world and our ability to shape it for the better. The right choice depends on where your passion and curiosity lead you!
FAQs
Polymers are large molecules composed of repeating monomers linked together in chains, while macromolecules encompass molecules with thousands of atoms, including proteins and nucleic acids.
Polymers consist of linear or branched chains of monomers, whereas macromolecules exhibit complex three-dimensional structures often folded intricately.
Polymers include plastics, synthetic fibers, and rubber. Macromolecules encompass proteins, nucleic acids (DNA, RNA), and complex carbohydrates.
Polymers are primarily held together by covalent bonds between monomers. Macromolecules involve covalent and non-covalent bonds, such as hydrogen bonds, influencing their unique structures and functions.
Yes, both polymers and macromolecules can be found in natural and synthetic forms. Polymers include both natural substances like rubber and human-engineered materials like plastics. Macromolecules comprise natural proteins and nucleic acids as well as synthetic counterparts designed for specific applications.
Polymers possess diverse properties tailored for various uses, such as flexibility, elasticity, and strength. Macromolecules exhibit specific functions driven by their intricate folding patterns and molecular interactions.
Polymers find applications in packaging, textiles, and medical devices. Macromolecules are crucial for biological functions, including enzyme reactions, genetic information storage, and gene therapies.
Quantum effects impact both polymers and macromolecules, influencing properties, behavior, and interactions at the molecular level. In polymers, they determine properties like electrical conductivity, while in macromolecules, they contribute to stability and enzymatic reactions.
In polymers, intermolecular forces determine how chains interact and respond to stress. In macromolecules, these forces shape folding patterns and functions, with hydrogen bonds playing a crucial role.
Polymers are essential in creating nanocomposites for enhanced material properties. Macromolecules, especially artificial proteins and synthetic DNA, enable precise manipulation at the nanoscale, contributing to fields like drug delivery and nanostructure construction.
Read More:
Reference:
- “Polymer.” Wikipedia, Wikimedia Foundation, 5 Oct. 2018.
- “Macromolecule.” Wikipedia, Wikimedia Foundation, 9 Oct. 2018.
Contents