| Aspect | Polymers | Monomers |
|---|---|---|
| Definition | Large molecules composed of repeating monomer units. | Small molecules that serve as building blocks. |
| Size | Larger molecular weight; macroscopic structures. | Smaller molecular weight; microscopic units. |
| Structure | Often have repetitive, linear, or branched chains. | Have diverse and less repetitive structures. |
| Bonding | Covalent bonds hold monomers together in chains. | Can have covalent, hydrogen, or weak bonds. |
| Physical Properties | Varied properties based on composition and structure. | Simpler and limited physical properties. |
| Chemical Properties | Can be chemically modified and engineered. | Precise control over chemical structure. |
| Applications | Wide range of industrial and consumer applications. | Chemical synthesis, specialty chemicals. |
| Environmental Impact | Can be non-biodegradable; environmental concerns. | Varies depending on the specific monomer. |
| Scale of Production | Suitable for large-scale industrial production. | Often used in laboratory-scale or specialty. |
| Regulatory Considerations | Subject to regulations due to environmental impact. | Compliance with safety standards is essential. |
| Cost and Scalability | Economical for mass production and large quantities. | Costs may vary; suitable for smaller volumes. |
| Versatility and Customization | Highly versatile and can be customized for needs. | Offer precision in chemical synthesis. |
| Biodegradability | Many traditional polymers are not biodegradable. | Biodegradable monomers are being developed. |
| Emerging Trends and Innovations | Smart polymers, nanocomposites, biopolymers, etc. | Green chemistry, precision synthesis, etc. |
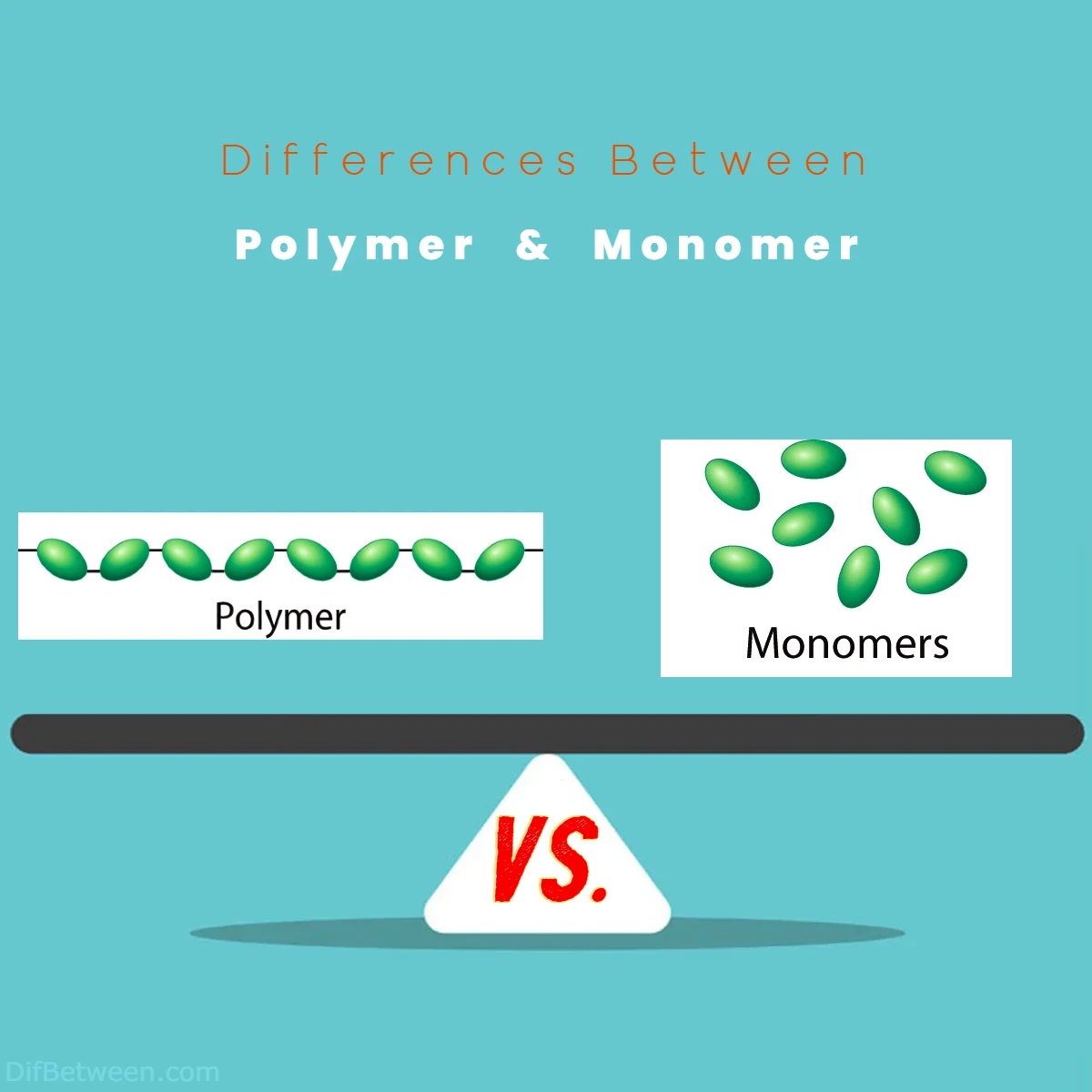
Polymers, those long chains of repeating monomer units, are the unsung heroes of our modern world, shaping everything from the clothes we wear to the cars we drive. Monomers, on the other hand, are the molecular architects, crafting the blueprint for these polymers. Together, they weave a mesmerizing tale of innovation and versatility, with countless applications that touch our lives daily.
Differences Between Polymer and Monomer
The main differences between Polymer vs Monomer lie in their molecular structures and functions. Polymers are large molecules formed by the repetitive bonding of smaller units, known as monomers, through strong covalent bonds. Polymers often exhibit diverse properties and are widely used in various industries due to their versatility. Monomers, on the other hand, are the individual building blocks from which polymers are constructed. They are smaller molecules with simpler structures and can serve as precise chemical tools for custom synthesis. In summary, the key distinctions revolve around size, structure, and functionality, with polymers being larger, more complex, and versatile, while monomers are smaller and provide precise control in chemical reactions.
Defining Polymers and Monomers
Polymers: Building Blocks of Matter
Polymers, derived from the Greek words “poly” (meaning many) and “meros” (meaning parts), are large molecules composed of repeating subunits known as monomers. Imagine a chain made up of countless identical links, and you have a basic understanding of a polymer. These chains can vary in size from just a few monomer units to thousands or even millions, giving rise to a diverse range of materials with unique properties.
One of the most iconic examples of a polymer is polyethylene, which is used to make plastic bags. Its structure consists of long chains of repeating ethylene monomer units (CH2=CH2). These chains are held together by covalent bonds, creating a flexible and versatile material.
Polymers are everywhere in our daily lives. From the rubber in your car tires to the proteins in your body, they play a pivotal role in shaping the world around us.
Monomers: The Molecular Building Blocks
Now, let’s meet the other half of our duo: monomers. These are the individual, smaller molecules that serve as the building blocks for polymers. Picture monomers as the Lego bricks that you assemble to create intricate structures.
Monomers come in various forms and types, each with its own unique properties and reactivity. They can be organic or inorganic, simple or complex. For instance, glucose is a monomer found in nature and is a component of the natural polymer cellulose, which makes up the cell walls of plants.
The Chemistry Behind Polymers and Monomers
Bonding in Polymers and Monomers
Polymer Bonding
The fundamental difference between polymers and monomers lies in their molecular structure and bonding. Polymers are characterized by strong covalent bonds that link the monomers together. In a covalent bond, atoms share electrons, creating a stable connection between them. This shared electron interaction is what holds the polymer chain intact.
For example, in the case of polyethylene, the carbon atoms in each ethylene monomer form covalent bonds with each other. This results in a long chain of carbon atoms, with hydrogen atoms attached to them. This interconnected structure gives polyethylene its strength and flexibility.
Monomer Bonding
Monomers, on the other hand, can have different types of bonding. Some monomers also form covalent bonds, just like in polymers, while others can be held together by weaker forces such as hydrogen bonds or van der Waals forces. The type of bonding in a monomer depends on its chemical composition and the atoms involved.
For instance, consider glucose again, a common biological monomer. Glucose molecules are held together by covalent bonds between carbon, hydrogen, and oxygen atoms. These covalent bonds create a stable, ring-like structure.
Molecular Structure
Polymer Structure
Polymers have a repetitive and often linear or branched structure. This repetitive nature is a consequence of the repeating monomer units. The arrangement of monomers and the length of the polymer chain influence the material’s properties. Longer chains tend to be more flexible, while branched chains can create a more rigid structure.
For instance, high-density polyethylene (HDPE) has a more ordered and linear structure, which gives it strength and rigidity. In contrast, low-density polyethylene (LDPE) has a more branched structure, making it more flexible.
Monomer Structure
Monomers have a less repetitive and more diverse structure compared to polymers. They can be simple molecules like ethylene (C2H4), which consists of two carbon atoms and four hydrogen atoms, or more complex structures like amino acids, which are the monomers of proteins.
The diversity in monomer structure is what allows for the vast variety of polymers with different properties and applications. By combining different monomers, chemists can design polymers tailored to specific needs, such as creating heat-resistant plastics or biodegradable materials.
Formation: Polymerization vs. Monomer Isolation
Polymerization: Building Large from Small
Polymerization is the process through which monomers are chemically linked together to form polymers. This reaction typically involves breaking or sharing of chemical bonds between monomers and the formation of new covalent bonds that connect them.
Polymerization can occur through various mechanisms, depending on the type of polymer being produced. Some common types of polymerization reactions include:
- Addition Polymerization: In this process, unsaturated monomers, like ethylene, undergo a series of addition reactions, where the double bonds break and new single bonds form. This results in a long, linear polymer chain.
- Condensation Polymerization: This type of polymerization involves the elimination of small molecules, such as water or methanol, as byproducts during the formation of covalent bonds between monomers. An example of condensation polymerization is the formation of nylon.
- Free Radical Polymerization: Initiators, such as peroxides, generate free radicals that initiate chain reactions between monomers. This process is commonly used to produce synthetic rubbers and plastics.
Monomer Isolation: Unveiling the Building Blocks
While polymerization brings monomers together to form large, complex structures, monomer isolation involves extracting or isolating individual monomers from a polymer. This process can be crucial for recycling and reusing materials, as well as for studying the properties of specific monomers.
Monomer isolation techniques depend on the type of polymer and the desired outcome. Common methods include:
- Depolymerization: This involves breaking the covalent bonds between monomers to reverse the polymerization process. For example, PET (polyethylene terephthalate) can be depolymerized into its constituent monomers, ethylene glycol, and terephthalic acid.
- Hydrolysis: In cases where water-sensitive polymers are involved, hydrolysis can be used to cleave the polymer chains into monomers. Cellulose, a natural polymer, can be hydrolyzed to produce glucose monomers.
- Thermal or Chemical Methods: High-temperature processes or chemical treatments can be employed to break down polymers into their monomeric units. This is often used in the recycling of plastics.
Properties of Polymers and Monomers
Physical Properties
Polymers
The physical properties of polymers are diverse and depend on factors such as the type of monomers used, the polymerization process, and the molecular weight of the polymer. Some common physical properties of polymers include:
- Density: Polymers can have varying densities, with some being lightweight and others more dense. For example, polypropylene is lightweight, while polyvinyl chloride (PVC) is denser.
- Mechanical Strength: Polymers can be engineered to have a wide range of mechanical properties, from flexible and elastic to rigid and tough. This makes them suitable for applications ranging from soft rubbery materials to hard plastics.
- Melting Point: The melting point of polymers varies greatly. Some polymers have low melting points, making them easy to process, while others have high melting points, making them suitable for high-temperature applications.
Monomers
Monomers, being the building blocks of polymers, are typically small molecules with relatively simple physical properties. They may exist as gases, liquids, or solids at room temperature, depending on their chemical structure.
For example, ethylene, a gaseous hydrocarbon, serves as a monomer for polyethylene, a solid polymer. The physical state and properties of a monomer largely influence the characteristics of the resulting polymer.
Chemical Properties
Polymers
Polymers exhibit a wide range of chemical properties depending on their composition. Some notable chemical properties of polymers include:
- Chemical Inertness: Many polymers are resistant to chemical reactions and do not readily react with acids, bases, or other chemicals. This makes them suitable for storing and transporting various substances.
- Chemical Reactivity: While most polymers are chemically inert, some can be engineered to be highly reactive. For example, certain polymers are designed to absorb and release specific chemicals, making them useful in drug delivery systems.
- Compatibility: Polymers can be chemically modified to enhance their compatibility with other materials. This is important in industries such as coatings, adhesives, and composites.
Monomers
Monomers, being smaller and simpler molecules, tend to be more chemically reactive than polymers. They readily participate in chemical reactions, making them versatile building blocks for the synthesis of various polymers.
For example, the double bond in the ethylene monomer is highly reactive, allowing it to undergo addition polymerization to form polyethylene. Monomers can also be modified or functionalized to introduce specific chemical properties into the resulting polymer.
Applications of Polymers and Monomers
Polymers in Everyday Life
Polymers have infiltrated nearly every aspect of our daily lives. From the moment we wake up until we go to bed, we encounter and rely on polymer materials. Here are some common applications:
| Application | Polymer | Properties |
|---|---|---|
| Packaging | Polyethylene, Polypropylene | Lightweight, flexible |
| Clothing | Polyester, Nylon | Durable, moisture-wicking |
| Medicine | Polystyrene, Polyethylene Glycol | Biocompatible, sterile |
| Electronics | Polyimide, Polycarbonate | Insulating, heat-resistant |
| Transportation | Polyurethane, Epoxy Resin | Impact-resistant, durable |
Monomers in Chemical Synthesis
Monomers serve as the foundation for the creation of numerous synthetic materials, chemicals, and pharmaceuticals. Their versatility in chemical reactions enables the production of a wide range of products. Here are a few examples:
- Pharmaceuticals: Monomers are used in the synthesis of drugs and pharmaceuticals. For instance, the monomer 2-ethylhexyl acrylate is employed in the production of certain medications.
- Plastic Additives: Monomers can be used to modify and enhance the properties of plastics. For example, styrene is a monomer used to improve the rigidity of polystyrene, commonly found in disposable cutlery and packaging materials.
- Chemical Intermediates: Monomers are often used as intermediates in chemical reactions to produce various compounds. Ethylene, for instance, is a key intermediate in the production of ethanol and ethylene oxide.
Environmental Impact
Polymers and the Environment
The environmental impact of polymers has become a significant concern due to their widespread use and persistence in the environment. While polymers offer many benefits, such as lightweight packaging and durable materials, they also pose challenges related to disposal and recycling.
Recycling
Recycling is a crucial aspect of managing polymer waste. Many polymers, including polyethylene terephthalate (PET) and polypropylene (PP), can be recycled into new products. The recycling process typically involves:
- Collection: Gathering used polymer products, such as bottles or containers.
- Sorting: Separating different types of polymers to prevent contamination.
- Cleaning: Removing contaminants, labels, and adhesives.
- Processing: Shredding and melting the polymer to create new materials.
- Manufacturing: Using recycled materials to produce new products.
Recycling reduces the demand for virgin polymers and minimizes the environmental impact of polymer production. However, challenges remain, such as ensuring proper collection and processing of recyclable materials.
Biodegradability
Another approach to addressing the environmental impact of polymers is developing biodegradable polymers. These materials can break down into natural compounds under certain conditions. Examples include polylactic acid (PLA) and polyhydroxyalkanoates (PHA).
Biodegradable polymers offer a potential solution for single-use plastic products, as they can degrade in composting facilities or natural environments. However, their widespread adoption and scalability are still areas of ongoing research and development.
Monomers and the Environment
Monomers themselves can have environmental implications, particularly when they are hazardous or persistent in the environment. Some key considerations include:
Toxicity
Certain monomers can be toxic to humans and the environment. For example, vinyl chloride, a monomer used in the production of polyvinyl chloride (PVC), is considered a carcinogen. Proper handling and disposal of such monomers are essential to minimize health and environmental risks.
Persistence
Some monomers can persist in the environment for extended periods, leading to pollution concerns. Polychlorinated biphenyls (PCBs), for example, are a group of synthetic monomers known for their environmental persistence and toxicity. Regulations and remediation efforts are in place to address the legacy of PCB contamination.
Advanced Concepts in Polymer Chemistry
Copolymers: Mixing It Up
While the term “polymer” often conjures images of long chains with identical monomer units, the polymer world is more diverse than you might think. Copolymers are a prime example of this diversity. They consist of two or more different types of monomers that are chemically bonded together in a single polymer chain.
Copolymers come in various architectures, depending on how the different monomers are arranged:
- Random Copolymers: Monomers are randomly distributed along the polymer chain, resulting in a sequence of varying monomer units.
- Block Copolymers: Monomers are grouped together in blocks along the chain. For example, a block copolymer might have a segment of monomer A followed by a segment of monomer B.
- Graft Copolymers: Monomers of one type are attached as branches to the polymer backbone consisting of another type of monomer. This structure resembles a tree with branches stemming from a trunk.
Copolymers offer a wide range of properties and applications. For instance, styrene-butadiene rubber (SBR) is a copolymer of styrene and butadiene, known for its excellent abrasion resistance and toughness, making it suitable for tire manufacturing.
Cross-Linking: Strengthening the Network
In some polymers, additional chemical bonds can form between polymer chains, creating a three-dimensional network. This process is known as cross-linking, and it plays a significant role in enhancing the mechanical and thermal properties of polymers.
Cross-linked polymers are often referred to as thermosetting polymers because they become permanently rigid and heat-resistant once cross-linked. This property makes them ideal for high-temperature applications and as insulating materials.
One of the most well-known cross-linked polymers is epoxy resin, used in a wide range of applications, from adhesives and coatings to composite materials. Epoxy resin consists of two components: a resin and a hardener. When mixed together, a chemical reaction occurs, forming strong cross-links between polymer chains.
Polymer Characterization Techniques
Understanding and characterizing polymers at the molecular level is essential for designing materials with specific properties. Several techniques are employed to analyze polymers and gain insights into their structure, composition, and properties. Some of these techniques include:
- Spectroscopy: Various spectroscopic methods, such as infrared spectroscopy (IR) and nuclear magnetic resonance (NMR) spectroscopy, are used to study the chemical structure and bonding within polymers.
- Gel Permeation Chromatography (GPC): GPC is a technique for measuring the molecular weight distribution of polymers, which is crucial for predicting their properties.
- Differential Scanning Calorimetry (DSC): DSC measures the heat flow associated with thermal transitions in polymers, providing information about their melting and crystallization behavior.
- X-ray Diffraction (XRD): XRD is used to analyze the crystalline structure of polymers and determine the degree of crystallinity.
- Rheology: Rheological tests assess the flow and deformation behavior of polymers, helping researchers understand their mechanical properties and processability.
Emerging Trends and Innovations
The world of polymers and monomers is in a constant state of evolution, driven by scientific discoveries and technological advancements. Here are some emerging trends and innovations in this field:
Smart Polymers
Smart polymers, also known as stimuli-responsive polymers or smart materials, are designed to respond to external stimuli such as temperature, pH, light, or electrical fields. These polymers can change their properties, shape, or behavior in response to specific triggers. They have applications in drug delivery, sensors, and adaptable materials.
One example of a smart polymer is shape-memory polymer, which can “remember” a specific shape and return to it when stimulated, making it useful in minimally invasive medical devices and self-healing materials.
Biopolymers
As sustainability becomes a pressing global concern, the use of biopolymers is gaining momentum. These are polymers derived from renewable resources, such as plant starch, cellulose, or proteins. Biopolymers have applications in biodegradable packaging, agricultural films, and even as a substitute for traditional plastics.
One well-known biopolymer is polylactic acid (PLA), which is derived from corn starch and used in 3D printing, food packaging, and medical implants.
Nanocomposites
Nanocomposites are materials in which nanoscale particles, such as nanoparticles or nanofibers, are dispersed within a polymer matrix. These nanoscale additives can significantly enhance the mechanical, thermal, and barrier properties of polymers.
For instance, adding nanoclay particles to polymer matrices improves their strength and flame resistance. Nanocomposites are utilized in automotive parts, aerospace components, and food packaging.
Monomers in Organic Synthesis
Beyond their role in polymerization, monomers are pivotal in organic synthesis, serving as precursors for a vast array of chemical compounds. Chemists leverage monomers to build complex molecules and develop new drugs, materials, and specialty chemicals.
Monomers in Drug Discovery
The field of drug discovery heavily relies on the synthesis of diverse chemical compounds, many of which begin as monomers. Medicinal chemists design and synthesize monomeric building blocks that can be assembled into complex molecules with specific biological activities.
For instance, the monomer piperidine serves as a key building block in the synthesis of numerous pharmaceuticals, including antipsychotics and analgesics.
Monomers in Polymer Modification
Monomers are not only used to create new polymers but also to modify existing ones. Functional monomers, which contain specific functional groups, can be copolymerized with other monomers to introduce desired properties into polymers.
For example, the incorporation of acrylic acid as a functional monomer into a polymer can impart improved adhesion, water resistance, and reactivity.
Monomers in Specialty Chemicals
The chemical industry relies on monomers as starting materials for producing a wide range of specialty chemicals, including solvents, surfactants, and additives. These chemicals play vital roles in various industrial processes and consumer products.
An example is ethylene oxide, which is a versatile monomer used to manufacture ethylene glycol (used in antifreeze and polyester fibers), nonionic surfactants (found in detergents), and various specialty chemicals.
Environmental Considerations in Polymer Chemistry
The environmental impact of polymers and monomers continues to be a topic of concern and innovation. Researchers and industry professionals are exploring several avenues to mitigate the environmental footprint of these materials.
Green Chemistry
Green chemistry principles advocate for the design of chemical products and processes that minimize the use of hazardous substances and reduce waste. In the context of polymers and monomers, green chemistry aims to develop more sustainable synthesis routes, use renewable feedstocks, and reduce the generation of harmful byproducts.
For example, researchers are investigating the use of renewable feedstocks like plant-based sugars as starting materials for polymer synthesis, reducing reliance on petrochemical sources.
Recycling Advances
Efforts to improve polymer recycling methods are ongoing. Innovations in mechanical recycling, which involves reprocessing plastics into new materials, are making recycling more efficient and economically viable. Additionally, advancements in chemical recycling techniques are being explored to break down polymers into their constituent monomers for reuse.
Chemical recycling processes aim to address challenges such as mixed plastic waste streams and the recycling of complex polymers.
Biodegradable Polymers
The development of biodegradable polymers continues to gain traction. These polymers are designed to break down into environmentally benign substances under specific conditions, reducing their persistence in the environment.
Research is focused on improving the properties and scalability of biodegradable polymers to offer viable alternatives to traditional plastics.
FAQs
A polymer is a large molecule composed of repeating subunits called monomers. These subunits are chemically bonded together, forming a chain or network-like structure.
A monomer is a small molecule that serves as the basic building block for polymers. These molecules can link together through chemical reactions to form larger polymer chains.
Polymers are typically much larger than monomers. Polymers consist of multiple monomer units, making them macroscopic in size, while monomers are microscopic and singular.
Polymers are held together by covalent bonds between the monomer units. These bonds involve the sharing of electrons, creating a strong connection.
Yes, there are various types of polymers, including addition polymers, condensation polymers, and copolymers, each with distinct characteristics and applications.
Polymers are used in a wide range of applications, such as plastics, textiles, packaging, medical devices, and automotive components.
Polymers are highly versatile due to their ability to be tailored for specific properties, while monomers provide precision in chemical synthesis, allowing for controlled modifications.
Polymers can have environmental concerns, especially non-biodegradable plastics. However, efforts are ongoing to develop eco-friendly polymers. Monomers’ environmental impact varies depending on the specific molecule.
Polymers are typically more suitable for large-scale industrial production due to their versatility and cost-effectiveness.
The choice depends on factors like desired properties, scale of production, environmental considerations, and regulatory requirements. Carefully evaluate these factors to make an informed decision.
Read More:
Contents
- Differences Between Polymer and Monomer
- Defining Polymers and Monomers
- The Chemistry Behind Polymers and Monomers
- Formation: Polymerization vs. Monomer Isolation
- Properties of Polymers and Monomers
- Applications of Polymers and Monomers
- Environmental Impact
- Advanced Concepts in Polymer Chemistry
- Emerging Trends and Innovations
- Monomers in Organic Synthesis
- Environmental Considerations in Polymer Chemistry
- FAQs