| Criteria | Isolated System | Closed System |
|---|---|---|
| Matter Exchange | Does not exchange matter with the surroundings | Does not exchange matter with the surroundings |
| Energy Exchange | Does not exchange energy with the surroundings | Allows energy exchange with the surroundings |
| Mass Conservation | Total mass remains constant | Total mass remains constant |
| Energy Conservation | Total energy remains constant | Total energy can change due to energy exchange |
| Boundary Flexibility | Rigid, impermeable boundaries simplify analysis | Flexible boundaries accommodate energy transfer |
| Thermodynamic Processes | Focuses on internal energy changes | Includes heat transfer, work, and other processes |
| Mathematical Modeling | Simplified models due to limited variables | More complex models due to energy exchange |
| Heat Transfer | No heat transfer due to energy isolation | Heat transfer considered for energy interactions |
| Practical Applications | Limited practical applications due to isolation | Widely used in various natural and human processes |
If you’ve ever wondered how matter and energy interact within physical boundaries, this is your chance to dive into the fascinating world of physics and engineering. These two systems may sound similar, but they harbor distinctive characteristics that can shape our understanding of everything from the cosmos to everyday appliances.
Differences Between Isolated System and Closed System
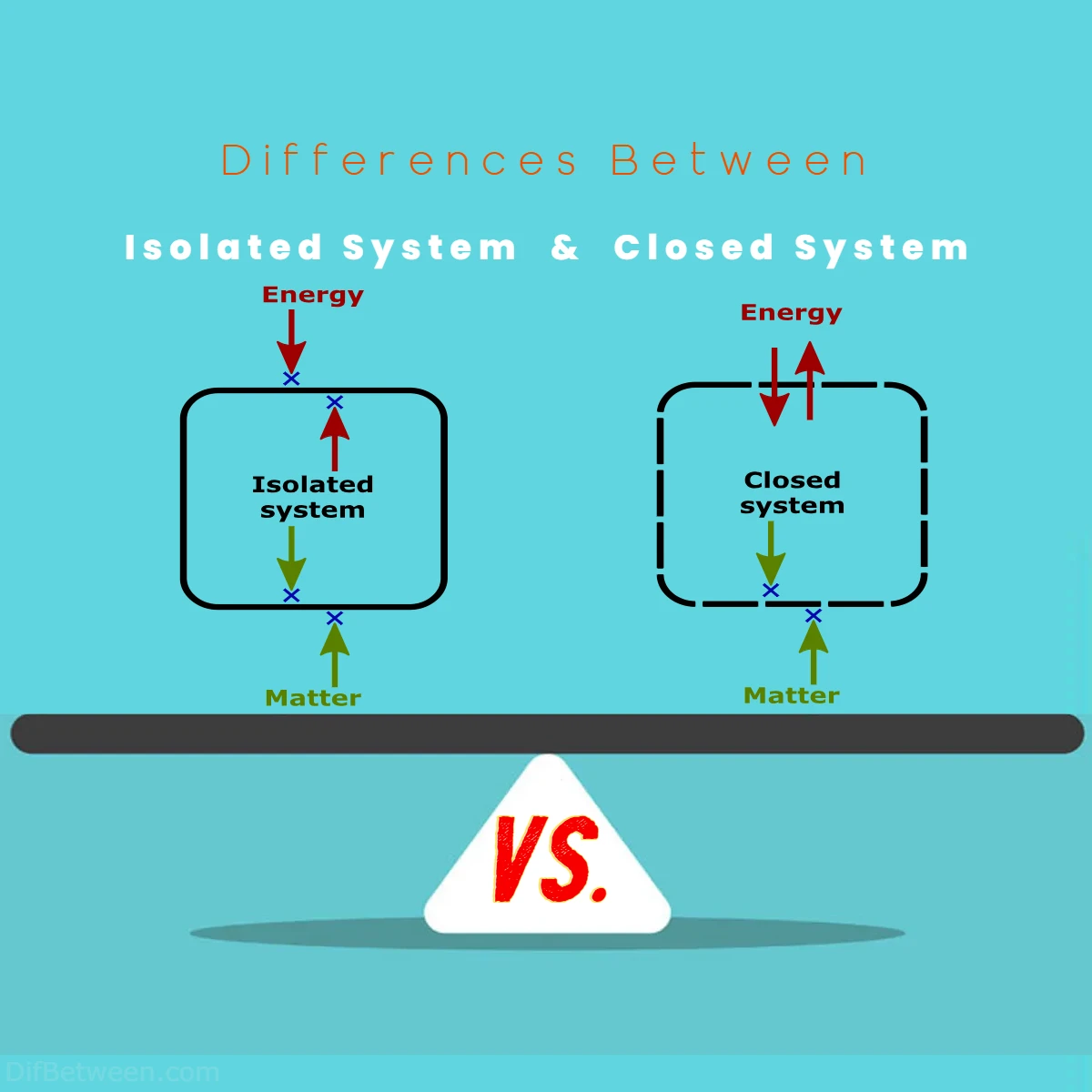
The primary differences between an isolated system and a closed system lie in their treatment of matter and energy exchange. In an isolated system, both matter and energy remain entirely sealed off from the surroundings, with no exchanges permitted. Conversely, a closed system allows energy transfer across its boundaries while maintaining a constant mass, making it a practical choice for real-world applications. These distinctions make isolated systems ideal for theoretical studies and closed systems indispensable for practical scenarios involving heat, work, and various natural and industrial processes.
Understanding the Basics
Isolated System
An isolated system is a physical system that does not exchange matter or energy with its surroundings. In simpler terms, it is a closed-off entity, completely sealed from its environment. This means that no mass or energy can flow in or out of an isolated system. In reality, truly isolated systems are rare, but they serve as valuable theoretical models in physics.
Closed System
A closed system, on the other hand, allows energy to flow in and out of the system boundaries, but it does not permit the exchange of matter with its surroundings. In a closed system, the total amount of matter remains constant, while energy can be transferred across its boundaries. Closed systems are more common in our daily experiences and can be found in various natural and engineered processes.
Now that we have the basic definitions in place, let’s explore the key differences between these two types of systems in more detail.
Table of Differences
To provide a quick overview, here’s a table highlighting the primary distinctions between isolated and closed systems:
| Criteria | Isolated System | Closed System |
|---|---|---|
| Matter Exchange | Does not exchange matter with the surroundings | Does not exchange matter with the surroundings |
| Energy Exchange | Does not exchange energy with the surroundings | Allows energy exchange with the surroundings |
| Mass Conservation | Total mass remains constant | Total mass remains constant |
| Energy Conservation | Total energy remains constant | Total energy can change due to energy exchange |
| Real-World Examples | The universe, a well-insulated thermos flask | A sealed container with a heating element |
| Theoretical Use | Valuable for theoretical studies in physics | Commonly used in practical applications |
| Practical Applications | Limited practical applications due to isolation | Widely used in various natural and human processes |
Now, let’s delve deeper into each of these differences to gain a more comprehensive understanding.
Matter Exchange
Isolated System
In an isolated system, matter exchange with the surroundings is entirely prohibited. This means that the system is completely sealed off from its environment, and no particles or substances can enter or exit. In a practical sense, finding a perfectly isolated system is exceptionally rare, as any physical system will have some level of interaction with its surroundings.
Closed System
In a closed system, like a sealed container with rigid walls, matter exchange is also restricted. However, unlike an isolated system, energy can still flow through its boundaries. This allows the system to maintain a constant mass while undergoing changes in energy due to heat transfer, for instance.
Energy Exchange
Isolated System
Isolated systems are known for their strict conservation of energy. They do not allow any energy exchange with the external environment. This implies that the total energy within an isolated system remains constant over time. This concept is famously encapsulated in the first law of thermodynamics, which states that the energy within an isolated system can neither be created nor destroyed; it can only change forms.
Closed System
Closed systems, in contrast, permit the exchange of energy across their boundaries. This exchange of energy can occur in various forms, including heat, work, and radiation. While matter remains constant in a closed system, its energy content can vary as energy is transferred to or from the surroundings.
Mass Conservation
Isolated System
Both isolated and closed systems share the characteristic of mass conservation. In an isolated system, the total mass remains unaltered, as no matter can enter or leave. This principle is upheld in theoretical physics and helps scientists make predictions about systems like the universe, where matter exchange with the outside is negligible.
Closed System
Similar to isolated systems, closed systems also adhere to the law of mass conservation. The total mass within a closed system remains constant, even though energy may flow across its boundaries. This conservation of mass is a fundamental principle in chemistry and engineering, where closed systems are frequently encountered.
Energy Conservation
Isolated System
Energy conservation is a hallmark feature of isolated systems. As previously mentioned, these systems do not exchange energy with their surroundings. Consequently, the total energy within an isolated system remains invariant. This concept is pivotal in understanding the behavior of celestial bodies and isolated physical experiments.
Closed System
Closed systems, while allowing energy exchange, still abide by the conservation of energy. The first law of thermodynamics is equally applicable to closed systems, ensuring that the total energy content is conserved, even though energy can be transferred across the system’s boundaries.
Real-World Examples
Isolated System
In the real world, finding a truly isolated system is an exceedingly rare occurrence. One often-cited example of an isolated system is the entire universe itself. While not perfectly isolated due to factors such as cosmic radiation, the universe is considered a valuable theoretical model of an isolated system for various physics studies.
Another example, although less grandiose, is a well-insulated thermos flask. When you pour hot coffee into a thermos, it remains hot for a considerable amount of time because the thermos is designed to minimize heat exchange with the surrounding environment, simulating the characteristics of an isolated system on a smaller scale.
Closed System
Closed systems are more commonly encountered in our daily lives and in scientific experiments. A classic example is a sealed container, such as a pressure cooker or a closed room with controlled environmental conditions. In these scenarios, matter does not escape or enter the system, but energy, in the form of heat or light, can still flow in and out. Practical applications of closed systems are widespread and include heating systems, chemical reactors, and even the Earth’s atmosphere to some extent.
Theoretical Use
Isolated System
Isolated systems are primarily employed for theoretical purposes in physics. They serve as idealized models to explore the fundamental principles of mass and energy conservation. The concept of an isolated system helps physicists make predictions about celestial bodies, cosmological events, and abstract physical experiments that closely align with the theoretical foundations of thermodynamics.
Closed System
Closed systems, while also valuable in theoretical discussions, find wider application in practical scenarios. Engineers, chemists, and biologists often work with closed systems to understand and design processes that involve energy exchange but need to maintain a constant mass. Closed systems offer a more realistic representation of many natural and human-made systems, making them crucial in various scientific and industrial fields.
Practical Applications
Isolated System
The practical applications of isolated systems are limited due to their idealized nature. In most real-world situations, some degree of matter or energy exchange occurs with the surroundings. Therefore, isolated systems are primarily confined to theoretical studies in physics and are not commonly used in practical engineering or industrial processes.
Closed System
Closed systems, with their flexibility in allowing energy exchange while preserving mass, are widely utilized in practical applications across numerous disciplines:
- Chemical Engineering: Chemical reactors are often designed as closed systems to control chemical reactions while managing energy inputs and outputs.
- Environmental Science: Earth’s climate system can be modeled as a closed system when analyzing energy flows and heat exchange processes.
- Biological Research: Laboratory incubators, where temperature and humidity are controlled, function as closed systems to create controlled environments for experiments.
- Heating and Cooling Systems: Home heating systems and air conditioners operate as closed systems, where heat is transferred but matter is conserved within the system.
In these and many other fields, closed systems play a pivotal role in understanding and optimizing processes.
Boundary Flexibility
Isolated System
An isolated system is characterized by an impermeable boundary, preventing any matter or energy exchange with the surroundings. This rigid boundary conceptually simplifies theoretical models but can be challenging to apply in real-world scenarios. As such, truly isolated systems remain largely theoretical constructs.
Closed System
Closed systems have more flexible boundaries. While they still prohibit matter exchange, the boundary allows for energy transfer. The flexibility of this boundary permits engineers and scientists to create closed systems that mimic real-world situations more closely. This feature is valuable for modeling and simulating dynamic processes.
Thermodynamic Processes
Isolated System
Isolated systems are often used to explore fundamental thermodynamic principles. In these systems, the focus is primarily on internal energy changes and the consequences of strict energy conservation. Isolated systems provide insight into concepts such as adiabatic processes, where no heat is exchanged with the surroundings.
Closed System
Closed systems are frequently encountered in thermodynamics and are integral to the study of heat and work interactions. In closed systems, the boundaries allow for heat exchange, which leads to the study of various thermodynamic processes like isothermal, isobaric, and isochoric processes. Understanding these processes is crucial in fields such as engineering and chemistry.
Mathematical Modeling
Isolated System
Mathematically modeling isolated systems can be more straightforward due to the strict constraints on matter and energy exchange. Equations governing the conservation of mass and energy are typically simpler, making theoretical predictions more manageable.
Closed System
Closed systems, with their energy transfer capabilities, often require more complex mathematical modeling. The introduction of heat and work interactions adds layers of intricacy to the equations used to describe these systems. Engineers and scientists employ sophisticated mathematical tools to analyze closed systems effectively.
Heat Transfer
Isolated System
In isolated systems, there is no mechanism for heat transfer since energy exchange with the surroundings is prohibited. This restriction simplifies the analysis but limits the applicability of isolated systems in scenarios where heat transfer is essential.
Closed System
Heat transfer is a central consideration in closed systems. These systems allow for the exchange of thermal energy with the surroundings. Understanding how heat flows in and out of a closed system is crucial for various applications, including designing efficient engines, managing thermal comfort in buildings, and optimizing industrial processes.
Practical Examples
Isolated System
In practical terms, finding true isolated systems is nearly impossible, as some level of interaction with the surroundings usually occurs. While the universe serves as a theoretical isolated system for cosmological studies, there are no known instances of isolated systems in everyday life or engineering applications.
Closed System
Closed systems have myriad practical applications:
- Heat Engines: Internal combustion engines, steam turbines, and refrigeration systems are all examples of closed systems where heat exchange plays a vital role in energy conversion.
- Chemical Reactions: Many chemical reactions are modeled as closed systems, where reactants and products are contained within a vessel, and heat may be exchanged to control reaction rates.
- Biological Systems: Organisms, cells, and ecosystems can be considered closed systems when analyzing energy flow and material cycling.
- Environmental Studies: Earth’s climate system, when studied from an energy perspective, is often treated as a closed system to understand heat transfer and radiative processes.
Isolated System or Closed System: Which One is Right Choose for You?
When it comes to studying physical systems, understanding whether to treat them as isolated or closed systems is a critical decision. Both approaches have their merits, and the choice depends on your specific goals, the nature of the system you’re dealing with, and the level of realism you aim to achieve. In this guide, we’ll help you make the right choice by exploring the factors that influence your decision.
Nature of the System
Isolated System
When to Choose an Isolated System:
- Theoretical Studies: If your primary goal is to explore fundamental physical principles and you want to simplify your analysis, an isolated system is a suitable choice. It allows you to focus on energy conservation and mass conservation without the complexity of energy exchange.
- Cosmological Research: In cosmology and astrophysics, where you’re dealing with celestial bodies and the universe at large, treating the universe itself as an isolated system is often the preferred approach. This simplifies calculations and theoretical models.
Closed System
When to Choose a Closed System:
- Practical Applications: In engineering, chemistry, biology, and other practical fields, closed systems are the norm. If your work involves real-world processes, equipment, or living organisms, using a closed system framework is essential to capture energy interactions accurately.
- Heat and Work Analysis: When your analysis requires a detailed examination of heat transfer and work interactions, as in thermodynamics, closed systems provide the necessary flexibility to account for these energy exchanges.
Goals of the Analysis
Isolated System
When to Choose an Isolated System:
- Energy Conservation Emphasis: If you’re primarily interested in studying the conservation of energy and its various forms without external influences, isolated systems are ideal for isolating energy changes within the system.
- Idealized Scenarios: In theoretical scenarios where you want to create simplified, idealized conditions to test hypotheses or explore abstract concepts, isolated systems offer the necessary isolation from the external environment.
Closed System
When to Choose a Closed System:
- Realistic Simulations: For practical simulations and experiments that aim to mimic real-world conditions as closely as possible, closed systems are indispensable. They allow for a more accurate representation of energy and matter exchanges.
- Engineering and Industrial Applications: Engineers and industrial practitioners often work with closed systems because they mirror the behavior of machines, chemical reactions, and biological processes in controlled environments.
Flexibility of Boundaries
Isolated System
When to Choose an Isolated System:
- Simplified Analysis: If you want to simplify your mathematical models and equations by dealing with rigid, impermeable boundaries, isolated systems provide this level of simplification.
- Conceptual Clarity: In educational settings, when introducing students to the principles of conservation of energy and mass, isolated systems help build a solid conceptual foundation.
Closed System
When to Choose a Closed System:
- Complex Processes: When you’re dealing with processes that involve dynamic interactions, such as heat flow, work done, or material exchange, the flexibility of closed system boundaries allows for a more comprehensive analysis.
- Engineering Design: Engineers often choose closed systems because they align with the real-world operation of machines, systems, and processes where energy transfer is essential.
Heat Transfer Considerations
Isolated System
When to Choose an Isolated System:
- No Heat Transfer: In scenarios where heat transfer plays no significant role and you want to focus solely on changes in internal energy, an isolated system simplifies the analysis.
Closed System
When to Choose a Closed System:
- Heat-Related Processes: If your analysis involves heat transfer, such as in engines, refrigeration systems, or thermal comfort studies, closed systems are necessary to account for these heat exchanges.
Mathematical Modeling Complexity
Isolated System
When to Choose an Isolated System:
- Simpler Models: If you prefer working with simpler mathematical models that do not require accounting for energy flow across boundaries, isolated systems are the way to go.
Closed System
When to Choose a Closed System:
- Complex Processes: When you’re dealing with complex processes where energy exchange is integral, such as chemical reactions or fluid dynamics, closed systems are essential despite the added mathematical complexity.
Conclusion
In the choice between treating a system as isolated or closed, your decision should align with your specific objectives, the nature of the system, and the level of detail required for your analysis. Isolated systems are valuable for theoretical explorations and scenarios where energy exchange is negligible, while closed systems are indispensable for practical applications, realistic simulations, and the study of dynamic energy interactions. Ultimately, understanding the nuances of each approach empowers you to make informed decisions that best serve your scientific or engineering endeavors.
FAQs
An isolated system is a physical system that does not exchange matter or energy with its surroundings. It is entirely sealed off, making it a theoretical model for studying mass and energy conservation.
A closed system is a physical system that does not exchange matter with its surroundings but allows energy transfer across its boundaries. This system is commonly encountered in practical applications.
Both isolated and closed systems do not exchange matter with the surroundings. The primary distinction lies in their treatment of energy exchange.
In an isolated system, there is no exchange of energy with the surroundings, resulting in constant total energy. In a closed system, energy transfer is allowed, leading to potential changes in the total energy content.
Isolated systems are ideal for theoretical studies in physics, where a strict conservation of energy and matter is desired, albeit rarely achieved in practice.
Closed systems find wide application in practical scenarios, including engineering, chemistry, biology, and environmental studies. They are valuable for modeling real-world processes.
Certainly! An isolated system can be likened to the entire universe, while a closed system can be exemplified by a sealed container with a heating element, like a pressure cooker.
Yes, both types of systems adhere to the principle of mass conservation, meaning the total mass within the system remains constant.
Isolated systems tend to result in simpler mathematical models due to the absence of energy exchange, while closed systems require more complex modeling to account for energy transfers.
Your choice between isolated and closed systems depends on your objectives. Use isolated systems for theoretical studies and closed systems for practical applications requiring energy interactions.
Read More:
Contents