| Aspect | Anode | Cathode |
|---|---|---|
| Electron Flow | Electrons flow out into the external circuit | Electrons flow in from the external circuit |
| Primary Reaction | Oxidation | Reduction |
| Electron Role | Donates electrons | Accepts electrons |
| Charge and Discharge | Releases electrons during discharging | Absorbs electrons during discharging |
| Electrode Role | Connects chemical reactions to circuit | Facilitates reduction reactions |
| Electrochemical Series | Tends to be higher up | Tends to be lower down |
| Energy Storage | Used in batteries to release energy | Used in batteries to store energy |
| Corrosion Protection | Sacrificial anodes protect structures from corrosion | Cathodic protection prevents corrosion |
| Electroplating | Provides ions for plating process | Acts as a site for deposition of plated material |
| Example Materials | Zinc, graphite, aluminum | Lithium cobalt oxide, copper, silver, graphite |
As we traverse the realm of electrons, reactions, and electrodes, you’ll discover that the anode and cathode are more than just polar opposites – they are the yin and yang of electrochemistry, each with its own distinct role and purpose. From the electrons flowing outwards from the anode to the electrons being embraced by the cathode, we’ll unravel the secrets of oxidation and reduction, the heartbeat of electrochemical reactions.
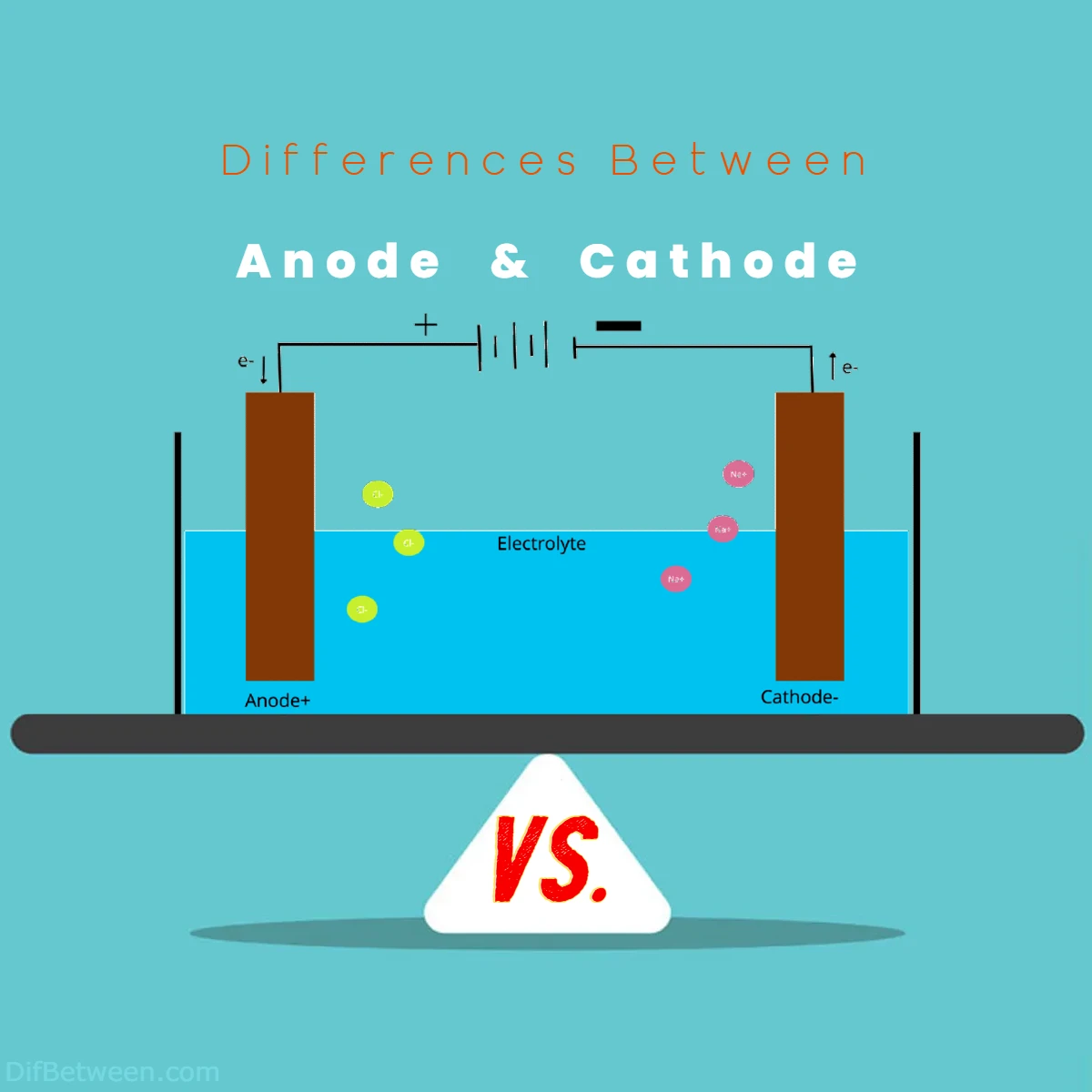
Differences Between Anode and Cathode
The main differences between anode and cathode lie in their roles within electrochemical reactions. An anode is where oxidation occurs, involving the loss of electrons, while a cathode is where reduction takes place, involving the gain of electrons. Electron flow direction sets them apart – at the anode, electrons exit into the external circuit, while the cathode welcomes electrons from the circuit. Functionally, anodes are electron donors, contributing to processes like energy storage and sacrificial protection. On the other hand, cathodes act as electron acceptors, playing a crucial role in rechargeable batteries, electroplating, and corrosion prevention.
The Basics: An Introduction to Anode and Cathode
Let’s begin our electrifying adventure with a basic understanding of what anode and cathode actually are.
Anode: The Launchpad of Electrochemical Reactions
Imagine the anode as a launching pad for chemical reactions powered by electricity. It’s where oxidation takes center stage. Oxidation, in simple terms, involves the loss of electrons, transforming substances into ions or compounds. In the world of batteries, the anode is where the magic happens. During discharging, it becomes a source of electrons, propelling electrical currents and enabling devices to function seamlessly.
Now, let’s delve a bit deeper. Anodes are not limited to a single role. In different contexts, various materials take up the mantle of being anodes. For instance, in a sacrificial anode system, a metal like zinc willingly corrodes to protect other metal components from a similar fate. In electroplating, anodes are vital in depositing metal layers onto surfaces with precision.
Cathode: The Hub of Reduction
Enter the cathode – the yin to the anode’s yang. While the anode facilitates oxidation, the cathode is where reduction dances its intricate steps. Reduction involves gaining electrons and the subsequent decrease in the oxidation state of a substance. This electrifying partner completes the circuit of electrochemical reactions. In batteries, the cathode welcomes electrons during charging, only to release them later when it’s time to power your devices.
The versatility of cathodes, like anodes, is quite astounding. Different applications demand different cathode materials. In rechargeable lithium-ion batteries, compounds like lithium cobalt oxide or iron phosphate swoop in as cathode heroes, shuttling ions back and forth with each charge-discharge cycle. In cathodic protection, this role reverses – the cathode becomes a protector, preventing corrosion of important structures.
Diving Deeper: The Key Differences
Having set the stage with our introductions, let’s now turn the spotlight on the key differences between anode and cathode.
1. Electron Flow Direction
Picture this: electrons are the lifeblood of electrochemistry. One of the primary distinctions between anode and cathode lies in the direction of electron flow.
- Anode: Here, electrons bid their fond farewell to the electrode and embark on a journey through the external circuit. Oxidation occurs at the anode, releasing electrons as chemical reactions unfold.
- Cathode: Electrons arriving from the external circuit are welcomed with open arms at the cathode. Reduction processes are the order of the day, as electrons participate in chemical reactions, causing ions to gain electrons.
2. Oxidation vs. Reduction
Ah, the eternal tug-of-war between oxidation and reduction. This dynamic duo forms the cornerstone of electrochemical reactions.
- Anode: As the hotspot for oxidation, the anode witnesses substances losing electrons. These electrons flow outwards, creating the much-needed electric current for various applications.
- Cathode: Reduction takes the spotlight at the cathode. Here, substances gain electrons, undergoing transformations that often result in the storage of energy, making it a pivotal player in the rechargeable battery universe.
3. Electron Donor vs. Electron Acceptor
Imagine the anode and cathode as partners in a grand ballroom dance of electrons. They each play a distinct role in this captivating performance.
- Anode: Known as the electron donor, the anode generously provides the electrons required for oxidation. It’s akin to a wellspring of energy, offering up its electrons for the greater good.
- Cathode: In contrast, the cathode elegantly embraces its role as the electron acceptor. It beckons electrons from the external circuit, participating in reduction reactions and creating a harmonious balance in the dance of electrochemistry.
4. Charge and Discharge
Batteries are a prime example of the interplay between anode and cathode during charge and discharge cycles.
- Anode: During discharge, the anode’s cupboard of electrons is flung open. As electrons flow out, the anode’s supply of ions decreases. However, during charging, the anode undergoes a transformation, welcoming back those electrons as ions journey back in.
- Cathode: For the cathode, discharge is a time of rest as it witnesses the influx of electrons. Ions move in, ready to engage in reduction reactions. Charging, on the other hand, signals a call to action as the cathode bids adieu to electrons, allowing ions to depart and store energy.
The Materials Game: Anode and Cathode Varieties
Now that we’ve uncovered the core disparities between anode and cathode, let’s explore the diverse array of materials that step into these roles.
Anode Materials
Anodes boast an impressive lineup of materials, each suited to specific applications:
- Metallic Anodes: In sacrificial anode systems, metals like zinc, aluminum, and magnesium selflessly corrode to protect other structures from degradation. Ships, pipelines, and underground tanks owe their durability to these sacrificial heroes.
- Graphite Anodes: Widely used in lithium-ion batteries, graphite anodes offer exceptional energy storage capabilities. They become home to lithium ions during charging, releasing them during discharging to power our devices.
- Platinum Anodes: These stalwarts of electroplating bring precision to the world of surface coatings. They ensure that metal ions in a solution are judiciously deposited onto surfaces, yielding products with impeccable finishes.
Cathode Materials
Cathodes also boast a diverse repertoire of materials, each tailored to a specific purpose:
- Lithium Cobalt Oxide: This cathode material is a rockstar in lithium-ion batteries. Its ability to release and absorb lithium ions during charge and discharge cycles makes it a cornerstone of portable electronics.
- Lithium Iron Phosphate: Renowned for its safety and longevity, this cathode material finds its home in electric vehicle batteries. It offers a stable structure and remarkable thermal resilience, ensuring the safety of the battery pack.
- Copper Cathodes: In electrorefining of copper, these cathodes emerge as pure copper havens. Through a meticulously controlled electrochemical process, impurities are left behind, resulting in high-purity copper sheets.
The Electrochemical Reactions: Unveiling the Mysteries
Let’s peel back the layers and venture into the heart of electrochemical reactions. This is where the true magic of anode and cathode differences comes to light.
Electrochemical Cell: A Dualistic Dance
An electrochemical cell is like a grand stage where anode and cathode each play their distinct roles in an electrifying dance of chemistry. Picture a battery, for instance. It comprises an anode and a cathode immersed in an electrolyte solution. This stage is set for a captivating performance of electron exchange and ion movement.
- Anode: Imagine the anode as the source of action. Here, chemical reactions occur that release electrons into the external circuit. These reactions often involve substances losing electrons and transforming into ions. The anode, in essence, is the catalyst for oxidation.
- Cathode: On the flip side, the cathode takes on the role of receiving the electrons. It’s where reduction reactions unfold, involving the gain of electrons by substances. These reactions often result in the storage of energy, making the cathode a pivotal player in battery systems.
Electrolysis: The Role Reversal
When it comes to electrolysis, the roles of anode and cathode do a fascinating flip. This process involves using electrical energy to drive a non-spontaneous chemical reaction. In this scenario, the anode and cathode swap their usual roles.
- Anode: During electrolysis, the anode becomes the site of oxidation. It’s where anions from the electrolyte migrate to lose electrons and undergo chemical changes.
- Cathode: The cathode, in turn, becomes the stage for reduction. Cations from the electrolyte converge here, gaining electrons and undergoing transformations under the influence of electrical energy.
The Essential Role of Electrodes: Anode and Cathode in Action
Let’s shift our focus to the essential role of electrodes in the world of anode and cathode distinctions. Electrodes are the gatekeepers that enable the flow of electrons and ions, turning the wheels of electrochemistry.
Anode as Electrode
In various electrochemical systems, the anode functions as an electrode, mediating the transfer of electrons. It’s the bridge that connects the chemical reactions happening within the cell to the external circuit. Anodes can be made from a variety of materials depending on the application:
- Metal Anodes: Often used in batteries, metal anodes serve as reservoirs of electrons that can be released during discharging. They form the interface where oxidation reactions take place, generating a flow of electrons.
- Inert Anodes: In certain cases, anodes need to be chemically stable and unreactive to the surrounding environment. Inert materials like graphite or certain types of metal oxides can be used as anodes in such scenarios.
Cathode as Electrode
Just like the anode, the cathode also assumes the role of an electrode, facilitating the flow of electrons and ions. It’s the site where reduction reactions take place, allowing the storage of energy or the deposition of metals:
- Active Cathodes: In rechargeable batteries, cathodes actively participate in the storage and release of ions, such as lithium ions in lithium-ion batteries. Materials like metal oxides or phosphates are often used as active cathode materials.
- Inert Cathodes: In electroplating, cathodes are used as passive surfaces where metal ions from a solution are reduced and deposited onto the surface, resulting in the formation of a metallic coating.
The Electrochemical Series: Where Do They Stand?
Enter the electrochemical series – a list of elements organized according to their standard electrode potentials. This series provides insights into the tendencies of elements to gain or lose electrons, shedding light on anode and cathode behavior.
Anode in the Electrochemical Series
Elements higher up in the electrochemical series have a greater tendency to lose electrons and thus function as anodes in electrochemical cells. These anodes readily undergo oxidation, making them crucial players in various applications:
- Zinc Anode: With a lower standard reduction potential, zinc easily loses electrons and is commonly used as a sacrificial anode to protect other metals from corrosion.
- Aluminum Anode: Aluminum follows suit as a sacrificial anode, offering its electrons to safeguard structures in environments where corrosion is a concern.
Cathode in the Electrochemical Series
Conversely, elements lower down the electrochemical series exhibit a stronger tendency to gain electrons and serve as cathodes. These materials shine as cathodes in diverse scenarios:
- Copper Cathode: With a positive standard reduction potential, copper takes center stage in electrorefining processes, where impurities are left behind as pure copper is deposited onto cathodes.
- Silver Cathode: Silver’s low reactivity and affinity for electrons make it an ideal candidate for cathodes in certain electroplating applications, yielding exquisite finishes.
Table: A Side-by-Side Comparison
To wrap up our exploration of anode and cathode differences, let’s present you with a handy table that lays out the distinctions in a clear and concise manner:
| Aspect | Anode | Cathode |
|---|---|---|
| Electron Flow | Electrons flow out into the external circuit | Electrons flow in from the external circuit |
| Reaction Type | Oxidation | Reduction |
| Electron Role | Donates electrons | Accepts electrons |
| Charge and Discharge | Releases electrons during discharging | Absorbs electrons during discharging |
| Electrode Role | Connects chemical reactions to circuit | Facilitates reduction reactions |
| Electrochemical Series | Tends to be higher up | Tends to be lower down |
Wrapping Up: A Harmonious Coexistence
And there you have it, fellow enthusiasts of the scientific world! We’ve journeyed through the domains of anode and cathode differences, from their essential roles in electrochemical cells to their varied materials and unique behaviors. As you embark on your own experiments or simply marvel at the wonders of electrochemistry around you, remember that the dance between anode and cathode is what powers the modern world – a harmonious coexistence of electrons and ions that keeps the energy flowing and innovation thriving.
FAQs
The fundamental distinction lies in their roles during electrochemical reactions. An anode is where oxidation occurs, involving the loss of electrons, while a cathode is where reduction takes place, involving the gain of electrons.
Electrons flow outwards from the anode into the external circuit, contributing to oxidation reactions. In contrast, at the cathode, electrons flow in from the circuit, driving reduction reactions.
Anode and cathode roles can indeed change depending on the context. For example, during electrolysis, the anode and cathode roles reverse compared to their typical functions in batteries.
Anodes are vital in batteries, releasing electrons during discharging. They’re also employed in sacrificial protection systems to prevent corrosion, as well as in electroplating processes to provide ions for coating surfaces.
Cathodes are essential in rechargeable batteries like lithium-ion ones. They store energy by facilitating the reduction of ions during charging and releasing that energy during discharging.
Certainly! Cathodic protection is a technique used to prevent corrosion in metal structures. By making the structure a cathode through the application of electrical current, it attracts corrosive agents, protecting the metal from degradation.
Absolutely! Different materials suit each role. For example, zinc and aluminum are often used as anodes in corrosion protection, while lithium cobalt oxide shines as a cathode in rechargeable batteries.
Anodes dissolve in electroplating solutions, providing metal ions that coat surfaces during the plating process. Cathodes, meanwhile, serve as the receiving surface where these ions are deposited, resulting in the desired finish.
Certainly! Anodes donate electrons, undergoing oxidation, while cathodes accept electrons, experiencing reduction. Anodes release electrons during discharging, while cathodes absorb them. These distinctions drive their roles in energy storage, corrosion protection, and various electrochemical processes.
For a comprehensive understanding, explore resources on electrochemistry, battery technology, and corrosion prevention. Engaging with educational websites, textbooks, and scientific journals will further enhance your knowledge of these fascinating concepts.
Read More:
Contents
- Differences Between Anode and Cathode
- The Basics: An Introduction to Anode and Cathode
- Diving Deeper: The Key Differences
- The Materials Game: Anode and Cathode Varieties
- The Electrochemical Reactions: Unveiling the Mysteries
- The Essential Role of Electrodes: Anode and Cathode in Action
- The Electrochemical Series: Where Do They Stand?
- Table: A Side-by-Side Comparison
- Wrapping Up: A Harmonious Coexistence
- FAQs