| Aspect | Titration | Back Titration |
|---|---|---|
| Approach | Direct and straightforward | Indirect, involving excess reactant |
| Timing | Endpoint determination during reaction | Endpoint determination after excess reactant titration |
| Ideal Scenarios | Well-defined endpoints, known solutions | Complex reactions, challenging endpoints |
| Precision vs. Practicality | Precision-focused for accuracy | Practical solution for challenging situations |
| Quantitative Brilliance | Precise determination of analyte concentration | Versatile analysis of substances with challenging reactions |
| Economical Insights | Affordable and minimal equipment requirements | Cost-effective through strategic use of excess reagent |
| Sample Complexity | Effective for uncomplicated sample matrices | Overcomes complexity and interferences in samples |
| Precision in Practicality | High precision with direct endpoints | Enhanced accuracy through multi-step analysis |
| Analytical Robustness | Dependable and robust for routine analyses | Adaptable method for complex or unconventional situations |
In the mesmerizing realm of chemistry, two fascinating techniques take center stage: Titration and Back Titration. These methods might sound like they belong to a scientific wizard’s toolkit, but fear not, for I’m here to unravel their mysteries and highlight the key differences between them. So, grab your lab coats and safety goggles as we embark on this illuminating journey!
Differences Between Titration and Back Titration
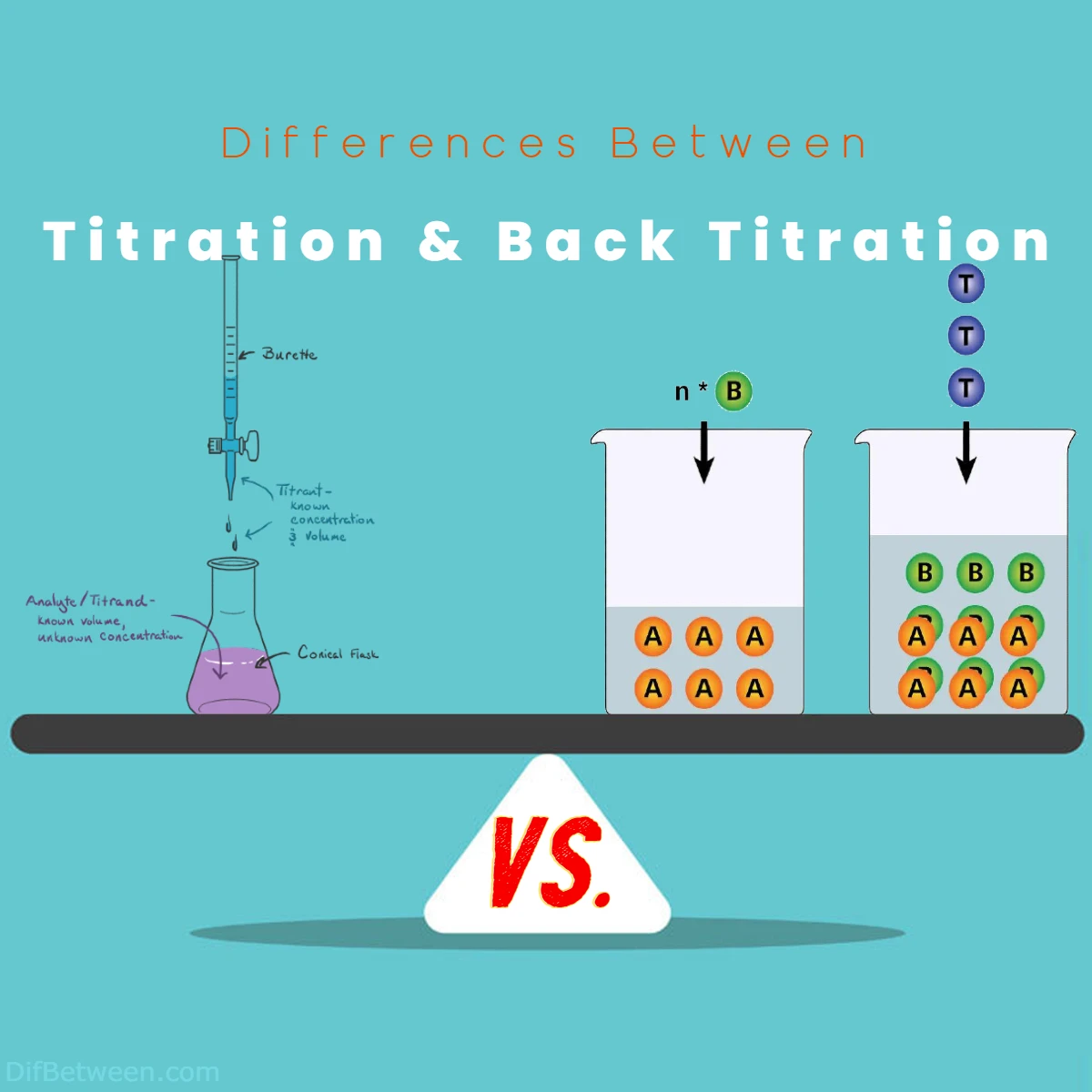
Titration and back titration are distinct techniques used in chemistry for quantitative analysis. The main difference lies in their approaches. Titration involves directly adding a solution of known concentration to an unknown solution until a reaction reaches completion, providing immediate results with well-defined endpoints. Conversely, back titration employs an excess of a known reagent to react with the unknown solution, followed by titration of the leftover excess. This indirect method is preferred in complex reactions or scenarios where the endpoint is challenging to detect. While titration offers precision and simplicity, back titration showcases adaptability and accuracy in navigating intricate chemical puzzles.
1. The Basics Unveiled
Titration: Imagine a delicate dance where two substances interact to reveal their hidden secrets. This dance is titration. In simple terms, it involves gradually adding a solution of known concentration to another solution until a chemical reaction reaches completion. By doing so, we can calculate the concentration of the unknown solution. Titration is like a masterful puzzle, where the pieces are the volumes and concentrations of the solutions, and the solution’s concentration we seek is the missing piece.
Back Titration: Now, picture a detective following footsteps back to unravel a complex case. This detective work is back titration. It’s a slightly more intricate process compared to titration. Instead of directly titrating the solution of interest, you react it with an excess of another reagent of known concentration. The excess reagent’s leftover amount is then titrated with a third solution to determine how much of it reacted initially. This information helps deduce the original solution’s concentration. Back titration is like taking a detour to arrive at the same destination, but with a twist of analytical brilliance.
2. The Methodical Approach
Titration: Ah, the elegance of simplicity! Titration involves a direct approach. You have your unknown solution, your known solution, and a clear endpoint – often marked by a color change. As you slowly introduce the known solution to the unknown, drop by drop, a chemical reaction silently unfolds. The moment the reaction is complete, the color change signals victory, and you can calculate the unknown solution’s concentration using stoichiometry.
Back Titration: Here comes the Sherlock Holmes of the lab world – back titration. When dealing with challenging scenarios where the reaction between the unknown solution and the titrant isn’t straightforward, back titration swoops in. By reacting the unknown solution with a known excess of another solution, you’re ensuring that no reactant is wasted, and every bit of the unknown solution reacts. This excess then provides a safety net for precise titration during the second stage, allowing you to work with smaller volumes and reducing errors.
3. Timing is Everything
Titration: In titration, timing is critical, much like the perfect moment to snap a photograph. The endpoint, often signaled by a color change or other observable change, signifies the conclusion of the reaction. Capturing this moment accurately is essential to obtaining precise results. This endpoint can be subtle, and a trained eye is crucial to recognizing it.
Back Titration: Timing gets even more interesting in back titration. Here, you’re not looking for an immediate color change. Instead, you’re gauging the leftover amount of the excess reactant. The precise titration of this excess determines how much of it reacted initially with the unknown solution. This delayed endpoint determination introduces an element of patience and precision, ensuring that you’re not missing any crucial data.
4. When to Choose Which?
Titration: Titration is like a swift arrow in your analytical quiver. Choose this technique when dealing with reactions that have a well-defined and easily observable endpoint. Acid-base titrations, where a pH indicator causes a sudden color shift, are classic examples. Similarly, when analyzing solutions of known concentrations, direct titration becomes a reliable choice.
Back Titration: Back titration is your strategic ally when facing complex scenarios. If your reaction kinetics are sluggish or the endpoint is tough to discern due to turbidity or other factors, back titration lends its helping hand. It’s also helpful when your unknown solution contains impurities that interfere with the direct titration.
5. Precision vs. Practicality
Titration: When accuracy is your guiding star, titration shines. This direct method minimizes errors associated with additional steps. The clear endpoint provides an instant signal that you’ve hit the bullseye, making titration a precision-focused choice.
Back Titration: Back titration, while slightly more convoluted, offers practicality in complex situations. It provides a workaround for challenging reactions and lets you salvage situations where direct titration might falter. It’s a strategic choice that sacrifices a bit of immediate precision for the sake of overall accuracy.
6. Quantitative Brilliance
Titration: In the realm of quantitative analysis, titration stands as a pillar of certainty. Its straightforward nature allows for precise determination of the concentration of the analyte (the substance being analyzed). The stoichiometric relationship between reactants ensures that each drop of the titrant (the solution of known concentration) corresponds to a specific amount of the analyte. This direct proportionality makes titration a favorite in quantitative chemical analysis.
Back Titration: In the realm of analytical flexibility, back titration emerges as a versatile technique. Its indirect approach permits the analysis of substances that might not react readily with the titrant in a direct titration. The surplus reagent adds a layer of control, making it possible to fine-tune the reaction conditions. This adaptability enables the determination of substances that may be difficult to quantify using other methods.
7. Economical Insights
Titration: Titration is a classic workhorse in the analytical laboratory, with a history that dates back centuries. Its simplicity and minimal resource requirements make it an economical choice. The equipment needed is basic – a burette, pipette, and appropriate glassware. This affordability has contributed to titration’s continued popularity in both educational settings and professional labs.
Back Titration: While slightly more intricate, back titration can also be economical when employed strategically. The use of excess reagent might seem wasteful at first glance, but it often proves to be a cost-effective solution. By ensuring the complete reaction of the analyte with the excess reagent, you’re safeguarding accuracy and reducing the need for additional analyses.
8. Sample Complexity
Titration: Titration thrives in the presence of uncomplicated sample matrices. When the analyte exists in a relatively pure form or in a matrix that doesn’t interfere with the reaction, direct titration shines. Acid-base titrations, for instance, are a prime example of this simplicity.
Back Titration: Enter back titration when the sample’s complexity throws a curveball. If impurities or interfering substances are present, direct titration might encounter stumbling blocks. Back titration’s two-step process, however, allows for careful handling of these interferences, enabling a clearer path to accurate results.
9. Precision in Practicality
Titration: Precision is the name of the game in titration. The simplicity of the method, combined with the ability to quickly and accurately reach the endpoint, yields results with high precision. This is especially valuable when dealing with solutions of known concentrations or reactions with distinct color changes as endpoints.
Back Titration: Precision gets a practical twist in back titration. While the initial titration might involve some excess of reagent, the subsequent titration of the excess provides an opportunity to fine-tune the results. This method ensures that the precise amount of excess reagent reacting with the analyte is determined, enhancing the accuracy of the analysis.
10. Analytical Robustness
Titration: Robustness is a hallmark of titration. Its direct approach and reliance on well-established reactions make it a dependable choice for routine analyses. The standard protocols and clear endpoints reduce the likelihood of errors, and well-trained analysts can consistently obtain accurate results.
Back Titration: Back titration embraces analytical robustness by navigating the challenges posed by complex reactions. Its adaptability allows analysts to overcome hurdles like slow reaction kinetics or elusive endpoints. By altering the conditions or reaction parameters, analysts can optimize the method to ensure accurate results even in unconventional scenarios.
| Aspect | Titration | Back Titration |
|---|---|---|
| Quantitative Precision | Direct proportionality enhances precision | Indirect method offers precision with adaptation |
| Economic Considerations | Minimal resource requirements | Strategically cost-effective approach |
| Sample Complexity | Thrives in uncomplicated matrices | Handles complex samples and interferences |
| Precision in Practice | High precision with distinct endpoints | Precision achieved through multi-step approach |
| Analytical Robustness | Dependable for routine analyses | Adaptable method for challenging situations |
As we conclude our expedition through the realm of titration and back titration, we unveil not just the differences, but also the unique strengths that each technique brings to the world of analytical chemistry. Titration, with its precision and simplicity, remains a steadfast choice for routine analyses. On the other hand, the adaptable nature of back titration opens doors to a world of complex scenarios, allowing analytical chemists to unlock answers that might otherwise remain hidden.
In the grand mosaic of scientific exploration, both techniques stand as pillars of knowledge, each offering its own distinct perspective. So, whether you’re following the direct path of titration or taking the scenic route of back titration, you’re embarking on a journey that celebrates the marvels of chemistry and the ingenuity of the human mind.
FAQs
The key difference lies in their approaches. Titration involves directly adding a solution of known concentration to an unknown solution until a reaction reaches completion. Back titration, on the other hand, employs an excess of a known reagent that reacts with the unknown solution, followed by titration of the leftover excess.
Opt for titration when dealing with reactions that have well-defined endpoints and solutions of known concentrations. It’s a precise method, especially suitable for routine analyses.
Back titration is a strategic choice for complex reactions, challenging endpoints, or scenarios where the direct titration might encounter obstacles due to slow reaction kinetics or interferences.
Titration offers high precision due to its straightforward nature and distinct endpoints. Back titration, while sacrificing some immediate precision, ensures accuracy by fine-tuning the analysis through its multi-step approach.
Both techniques can be cost-effective. Titration is known for its minimal equipment requirements. Back titration, while involving excess reagent, can be cost-effective when used strategically to achieve accurate results in challenging situations.
Titration is most effective with uncomplicated sample matrices and reactions that have observable endpoints. For complex samples or scenarios with interfering substances, back titration offers a solution by overcoming these challenges through its multi-step process.
In titration, the endpoint is reached during the reaction, often signaled by a color change. Back titration’s endpoint determination occurs after the excess reactant is titrated, introducing a delayed but more controlled endpoint determination.
Back titration’s indirect and multi-step approach makes it more adaptable to unconventional or challenging situations. It allows analysts to alter conditions and adapt the method for accurate analysis.
While less common, there could be scenarios where a preliminary titration provides initial information about the analyte’s concentration, followed by a back titration for a more accurate determination, especially if the reaction is intricate or the endpoint is difficult to observe.
Titration and back titration are valuable tools that enrich the field of analytical chemistry. They provide scientists with versatile methods to determine concentrations, overcome challenges, and uncover hidden insights within chemical reactions, expanding our understanding of the complex world of molecules.
Read More:
Contents






