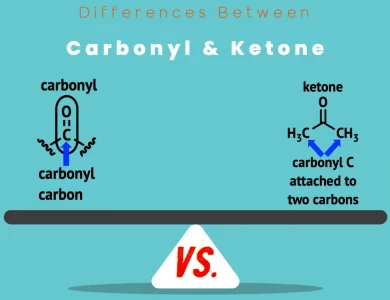
| Aspect | Alcohols | Phenols |
|---|---|---|
| Functional Group | -OH (Hydroxyl group) | -OH (Hydroxyl group) |
| Structural Makeup | Hydroxyl group attached to carbon atom (alkyl or aromatic) | Hydroxyl group directly bonded to aromatic ring (often benzene) |
| Acidity | Generally less acidic | Enhanced acidity due to resonance stabilization |
| Hydrogen Bonding | Forms hydrogen bonds with other alcohols and molecules | Can form hydrogen bonds, but limited due to aromatic ring |
| Boiling Points | Higher boiling points due to hydrogen bonding and London dispersion forces | Lower boiling points compared to alcohols of similar molecular weights |
| Reactivity | Versatile reactivity, including oxidation, dehydration, and substitution | Aromatic substitution reactions, acylation, and oxidation |
| Applications | Solvents, disinfectants, pharmaceuticals, personal care products | Antiseptics, plastics, adhesives, pharmaceuticals, antioxidants |
| Environmental Impact | Biodegradable, potential biofuel source from renewable biomass | Requires careful handling due to potential toxicity to aquatic life |
| Health Considerations | Ethanol safe in moderation, methanol highly toxic | Can be hazardous in concentrated forms, skin contact can cause burns |
| Medicinal Applications | Ethanol-based disinfectants, drug delivery | Potential health benefits due to antioxidant properties and medicinal potential |
| Safety Concerns | Methanol toxicity, potential for addiction with excessive consumption | Skin contact and inhalation can be harmful, ingestion can lead to poisoning |
Imagine donning a mental lab coat, equipped with a thirst for understanding and an insatiable curiosity. The stage is set, and the spotlight is on you, as we venture into the world of alcohols and phenols. These seemingly simple hydroxyl groups (-OH) lead us into a realm of complexity, where structures, reactivity, and applications intertwine. With each turn of the page, you’re not just reading – you’re participating in an intellectual exploration that unveils the heart of chemistry itself.
Differences Between Alcohols and Phenols
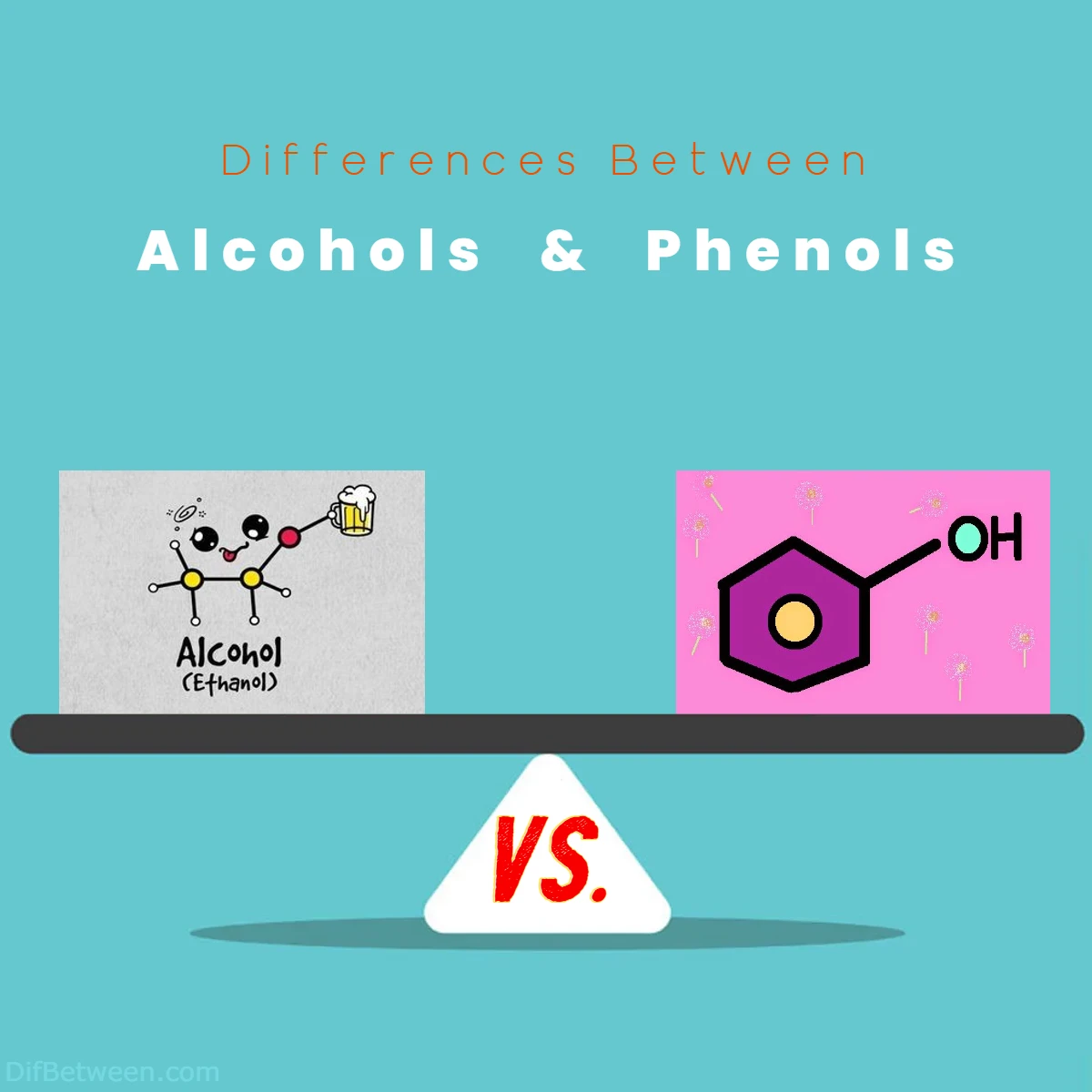
The main differences between alcohols and phenols lie in their structural arrangements and reactivity. Alcohols feature a hydroxyl (-OH) group attached to a carbon atom, while phenols showcase a hydroxyl group directly bonded to an aromatic ring, often a benzene structure. Notably, phenols possess enhanced acidity due to resonance stabilization, resulting in distinct reactivity patterns compared to alcohols. These differences impact their applications, with alcohols being versatile solvents and disinfectants, while phenols find use in areas like plastics, adhesives, and potential medicinal applications. Explore this comprehensive guide to delve deeper into the captivating distinctions between alcohols and phenols.
Structural Makeup: The Hydroxyl Connection
At a glance, alcohols and phenols might seem closely related due to their shared hydroxyl (-OH) group. This molecular arrangement, consisting of an oxygen atom bonded to a hydrogen atom, serves as the hallmark of both alcohols and phenols. However, it’s in the finer details of their structures that their differences emerge.
Alcohols: The Backbone of Organic Chemistry
Alcohols, often referred to as “hydroxy compounds,” form a versatile class of organic compounds. Their structure centers around a carbon atom (C) bonded to a hydroxyl group (-OH) through a single bond. The carbon atom in an alcohol can be part of an alkyl group (a chain of carbon atoms) or even an aromatic ring (benzene-based structure). This structural variability is a key characteristic of alcohols and contributes to their diverse properties and applications.
Alcohols are classified based on the nature of the carbon atom to which the hydroxyl group is attached. The primary, secondary, and tertiary distinctions are made based on the number of carbon substituents attached to the carbon bonded to the hydroxyl group. This classification impacts the reactivity and physical properties of alcohols, making them invaluable in fields such as pharmaceuticals, solvents, and organic synthesis.
Phenols: The Aromatic Hydroxy Compounds
Phenols, on the other hand, add a twist to the hydroxyl group story. While they share the same hydroxyl (-OH) group with alcohols, phenols are distinguished by the presence of an aromatic ring. This aromatic ring, often a benzene ring, lends phenols a unique set of properties and behaviors that set them apart from alcohols.
In phenols, the hydroxyl group is directly bonded to the aromatic ring, creating a distinct electronic environment. This arrangement influences the acidity of phenols compared to alcohols. Phenols tend to be more acidic due to the resonance stabilization offered by the aromatic ring, which helps disperse the negative charge of the deprotonated oxygen. This enhanced acidity has implications in various chemical reactions and biological processes where phenols play a crucial role.
Physical Properties: A Glimpse into Molecular Behavior
The differences in structural arrangements between alcohols and phenols give rise to distinctive physical properties that are important in various applications and industrial processes.
Alcohol’s Unique Traits
Alcohols exhibit a range of physical properties based on factors like molecular size, branching, and intermolecular forces. In general, alcohols tend to have higher boiling points compared to their alkane counterparts of similar molecular weight. This is due to hydrogen bonding – a type of intermolecular force that occurs between the hydrogen atom of the hydroxyl group in one molecule and the oxygen atom of another molecule.
As the size of the alkyl group attached to the hydroxyl-bearing carbon increases, the London dispersion forces (another type of intermolecular force) also come into play, impacting the boiling points. For instance, a tertiary alcohol with three alkyl groups attached to the hydroxyl-bearing carbon will have a higher boiling point than a primary alcohol with just one alkyl group.
Phenolic Peculiarities
Phenols, thanks to their aromatic ring and hydroxyl group arrangement, have distinct physical properties. Unlike alcohols, phenols don’t participate in intermolecular hydrogen bonding to the same extent. While they can form hydrogen bonds, the presence of the aromatic ring limits the potential for hydrogen bonding, resulting in lower boiling points compared to alcohols of similar molecular weights.
Furthermore, the enhanced acidity of phenols plays a role in their solubility behavior. Phenols are more soluble in aqueous solutions compared to hydrocarbons due to their ability to form hydrogen bonds with water molecules. This solubility is particularly pronounced for smaller phenols.
Chemical Reactivity: Unveiling Reaction Pathways
The distinct structural features of alcohols and phenols translate into diverse chemical reactivity profiles, enabling them to participate in a wide range of reactions.
Alcohol’s Transformations
Alcohols are known for their versatile reactivity, making them indispensable in organic synthesis. The hydroxyl group in alcohols can undergo various chemical transformations, including:
- Oxidation: Alcohols can be oxidized to form aldehydes or ketones using oxidizing agents like potassium dichromate. Primary alcohols can further oxidize to carboxylic acids. This oxidation process is the foundation for producing organic compounds like perfumes and flavorings.
- Dehydration: Under appropriate conditions, alcohols can lose a water molecule, resulting in the formation of alkenes. This dehydration reaction is significant in the petrochemical industry for producing fuels.
- Substitution: Alcohols can undergo substitution reactions where the hydroxyl group is replaced by another functional group. One example is the conversion of alcohols into alkyl halides through reactions with hydrogen halides.
Phenol’s Dynamic Chemistry
Phenols, with their aromatic backbone, exhibit distinct reactivity patterns compared to alcohols. The enhanced acidity of phenols influences many of their reactions, including:
- Electrophilic Aromatic Substitution: Phenols readily undergo electrophilic aromatic substitution reactions due to the electron-donating nature of the hydroxyl group. This allows for the introduction of various functional groups onto the aromatic ring.
- Acylation: Phenols react with acylating agents to form esters through acylation. This reaction is widely used in the synthesis of pharmaceuticals and fragrances.
- Oxidation: Phenols can be oxidized to form quinones, which are important in biological processes and also have applications in the dye industry.
Applications: Where Chemistry Meets Practicality
The distinctive properties and reactivity of alcohols and phenols pave the way for their diverse applications across industries.
Alcohol’s Industrial and Medicinal Uses
Alcohols find widespread use as solvents, both in industrial settings and research laboratories. Their ability to dissolve a wide range of organic and inorganic compounds makes them invaluable in chemical reactions, extractions, and cleaning processes. Ethanol, a type of alcohol, serves as an antiseptic and disinfectant due to its ability to denature proteins and disrupt cell membranes, making it a staple in healthcare settings.
In the pharmaceutical industry, alcohols are employed as vehicles for drug delivery and as key reagents in various synthetic processes. Alcohols are also found in cosmetics, perfumes, and personal care products due to their ability to dissolve aromatic compounds and enhance fragrance.
Phenol’s Remarkable Presence
Phenols play a pivotal role in a variety of applications, particularly as antiseptics and disinfectants. Their enhanced acidity and antimicrobial properties make them valuable in preserving medical instruments, treating wounds, and maintaining hygiene.
Furthermore, phenolic compounds find applications in the production of plastics, resins, and adhesives. The ability of phenols to polymerize leads to the formation of strong and durable materials. Bisphenol A, a well-known phenolic compound, is a building block in the production of polycarbonates and epoxy resins, widely used in everyday products like water bottles and coatings.
While both alcohols and phenols have valuable uses, it’s essential to consider their potential toxicity and safety concerns.
Alcohol’s Safe and Hazardous Aspects
Alcohol consumption by humans is widespread, particularly in the form of ethanol found in alcoholic beverages. Ethanol, when consumed in moderation, is generally considered safe. However, excessive alcohol consumption can lead to various health issues, including liver damage, addiction, and impaired cognitive functions.
Methanol, another type of alcohol, is highly toxic to humans and can cause severe health complications, including blindness and even death. It is often used as an industrial solvent and fuel, emphasizing the importance of proper handling and safety precautions.
Phenol’s Cautionary Notes
Phenols, especially in their concentrated form, can be toxic and irritating to human tissues. Skin contact with phenol can lead to burns and dermatitis. Inhaling phenol vapors can cause respiratory issues, and ingestion can result in severe poisoning. Hence, careful handling and proper protective measures are crucial when working with phenols.
In today’s environmentally conscious world, it’s essential to consider the impact of chemical compounds on ecosystems and human health. Alcohols and phenols are no exception, and understanding their environmental footprint is crucial.
Alcohol’s Biodegradability and Renewable Sources
One notable aspect of alcohols is their relatively high biodegradability. Ethanol, for instance, readily breaks down in the environment, minimizing its long-term impact. This property has led to the use of ethanol as a biofuel, reducing greenhouse gas emissions and dependence on fossil fuels.
Furthermore, alcohols can be derived from renewable sources such as plant biomass. Bioethanol, produced from crops like corn or sugarcane, serves as an eco-friendly alternative to traditional fossil fuels. This aligns with sustainable practices and contributes to reducing the carbon footprint associated with transportation and energy production.
Phenol’s Environmental Concerns
Phenolic compounds, especially in their concentrated forms, can pose challenges in terms of environmental impact. Phenols can be toxic to aquatic life, affecting aquatic ecosystems when released into water bodies. As a result, industrial processes involving phenols must be carefully managed to prevent contamination and minimize harm to the environment.
Efforts are being made to develop greener processes for the production and use of phenolic compounds. Sustainable extraction methods, recycling, and waste treatment technologies are being explored to mitigate the potential negative impacts of phenols.
Biological Significance: Health and Medicinal Applications
The differences between alcohols and phenols extend beyond their chemical properties, influencing their biological interactions and applications.
Alcohol’s Role in Medicine and Health
Alcohols have been used for centuries in medical and healthcare settings due to their antiseptic and disinfectant properties. Ethanol-based hand sanitizers and disinfectants play a crucial role in preventing the spread of infectious diseases. Additionally, alcohols are used as vehicles for drug delivery, facilitating the absorption of medications in various pharmaceutical formulations.
Alcohol consumption also has notable effects on human health. Moderate alcohol consumption has been associated with potential cardiovascular benefits, including improved heart health. However, excessive alcohol consumption can lead to addiction, liver damage, and increased health risks.
Phenol’s Medicinal and Natural Roles
Phenolic compounds have gained attention for their potential health benefits due to their antioxidant properties. Many natural sources of phenols, such as fruits, vegetables, and herbs, are rich in these compounds, which have been linked to reducing the risk of chronic diseases such as heart disease, cancer, and neurodegenerative disorders.
Moreover, phenolic compounds are being investigated for their potential anticancer properties. Certain phenols have demonstrated the ability to inhibit the growth of cancer cells and promote apoptosis, making them subjects of intense research in the field of oncology.
Future Trends: Innovations and Discoveries
The fields of organic chemistry, biochemistry, and material science continue to evolve, giving rise to new discoveries and innovative applications for alcohols and phenols.
Alcohol-Based Renewable Energy
Alcohols, particularly ethanol, continue to play a pivotal role in the transition toward renewable energy sources. Bioethanol, derived from plant biomass, is being explored as a sustainable alternative to fossil fuels. Researchers are working to improve the efficiency of bioethanol production processes, making them more economically viable and environmentally friendly.
Phenolic Compounds as Pharmaceuticals
Phenolic compounds’ unique chemical properties make them attractive candidates for drug discovery. Scientists are investigating the potential of phenols in developing novel pharmaceuticals with targeted mechanisms of action. The antioxidant and anticancer properties of certain phenols open up new avenues for therapeutic interventions and disease management.
Alcohols or Phenols: Which One is Right for You?
When it comes to selecting the right chemical compound for a specific application, factors such as structure, properties, reactivity, and intended use all come into play. Alcohols and phenols, despite their similarities, possess distinct characteristics that make them better suited for certain tasks. Let’s dive into the considerations that can help you decide whether alcohols or phenols are the right choice for your needs.
Application-Specific Properties
Understanding the unique properties of alcohols and phenols is crucial in determining which compound aligns with your intended application.
Alcohols: Versatile and Solvent-Friendly
Alcohols, with their diverse structures, serve as versatile solvents in various chemical processes. If your application involves dissolving organic or inorganic substances, alcohols might be your go-to choice. The ability of alcohols to form hydrogen bonds with other compounds enhances their solvent capabilities, making them efficient in dissolving a wide range of substances.
Furthermore, if you’re looking for a compound with potential antiseptic properties, certain alcohols like ethanol could fit the bill. Ethanol-based hand sanitizers and disinfectants are effective in neutralizing pathogens on surfaces and skin due to their denaturing effect on proteins and disruption of cell membranes.
Phenols: Aromatic and Acidic
Phenols, distinguished by their aromatic ring and enhanced acidity, offer a different set of properties. If your application requires a compound with enhanced acidity or if you’re aiming to influence the aromatic character of a reaction, phenols might be your choice. The resonance stabilization offered by the aromatic ring makes phenols more acidic than alcohols, allowing them to participate in unique reactions.
For instance, if you’re involved in organic synthesis and need a compound for electrophilic aromatic substitution reactions, phenols could be the preferred option due to their electron-donating nature. Additionally, the antioxidant properties of certain phenolic compounds make them attractive candidates for applications in the food and pharmaceutical industries.
Reactivity and Reaction Pathways
The reactivity of alcohols and phenols determines their suitability for specific chemical transformations.
Alcohol Reactivity: Oxidation and More
Alcohols are known for their diverse reactivity, particularly in oxidation and substitution reactions. If your application involves transforming alcohols into carbonyl compounds (aldehydes or ketones) through oxidation, alcohols are the way to go. Primary alcohols can further be oxidized to carboxylic acids, expanding their utility in synthesis.
Alcohols can also participate in dehydration reactions, forming alkenes under appropriate conditions. This property is essential in the petrochemical industry for producing fuels and polymers.
Phenol Reactivity: Aromatic Substitution and Beyond
Phenols, with their aromatic backbone, are especially suited for electrophilic aromatic substitution reactions. If your application requires introducing various functional groups onto an aromatic ring, phenols are the compounds of choice. Their electron-donating hydroxyl group enhances their susceptibility to substitution reactions, making them valuable in the synthesis of diverse compounds.
Moreover, phenols can undergo acylation reactions to form esters. This property finds applications in fragrance and pharmaceutical synthesis, offering a unique reactivity pathway.
Safety and Environmental Considerations
Balancing the benefits of a compound with safety and environmental concerns is paramount.
Alcohol Safety and Health Effects
Alcohol safety considerations vary depending on the type and intended use. Ethanol, commonly used in alcoholic beverages, is safe in moderation. However, excessive consumption can lead to health issues such as addiction and liver damage. Methanol, on the other hand, is toxic and poses severe health risks if ingested.
Phenol Safety and Environmental Impact
Phenols, particularly in concentrated forms, can be hazardous to human health and the environment. Skin contact with phenols can result in burns, and their release into water bodies can harm aquatic ecosystems. Proper handling and waste management are essential when working with phenols to minimize their impact.
Making the Right Choice
In the alcohols vs. phenols debate, the right choice depends on your application’s specific requirements and considerations.
Choose alcohols when:
- Versatile solvents are needed for dissolving a range of substances.
- Antiseptic properties are desired for disinfection and sanitation purposes.
- Oxidation and dehydration reactions are integral to your chemical processes.
Opt for phenols when:
- Enhanced acidity and participation in specific reactions are crucial.
- Aromatic substitution reactions are required for functional group introduction.
- Potential antioxidant properties are beneficial for your application.
By understanding the properties, reactivity, and safety aspects of alcohols and phenols, you can confidently make the right choice for your unique needs, ensuring the success and safety of your chemical endeavors.
FAQs
The primary distinction lies in their structural makeup. Alcohols have a hydroxyl (-OH) group attached to a carbon atom, which can be part of an alkyl chain or an aromatic ring. Phenols, on the other hand, have a hydroxyl group directly bonded to an aromatic ring, often a benzene structure.
Phenols exhibit enhanced acidity compared to alcohols. This is due to resonance stabilization offered by the aromatic ring in phenols, which helps disperse the negative charge of the deprotonated oxygen. As a result, phenols are more acidic and participate in unique reactions.
Alcohols find use as versatile solvents, disinfectants, pharmaceuticals, and in personal care products. Phenols are employed in antiseptics, plastics, adhesives, and potential medicinal applications due to their unique properties and reactivity.
Alcohols like ethanol are safe in moderation, but excessive consumption can lead to health issues. Methanol, another type of alcohol, is toxic. Phenols, especially in concentrated forms, can be hazardous to human health and the environment. Proper handling and safety precautions are essential when working with both compounds.
Alcohols like ethanol can be derived from renewable sources and have relatively high biodegradability. Phenols, however, can pose challenges due to potential toxicity to aquatic life. Efforts are being made to develop greener processes for phenol production and use.
Yes, certain phenolic compounds possess antioxidant properties and are being investigated for potential health benefits. Phenols are also studied for their potential roles in pharmaceuticals, particularly in anticancer research and drug discovery.
Phenols are well-suited for electrophilic aromatic substitution due to their enhanced reactivity. The presence of the hydroxyl group in phenols enhances their electron-donating nature, making them ideal for introducing functional groups onto aromatic rings.
Alcohols like ethanol continue to play a role in renewable energy as biofuels. Phenolic compounds are being explored for pharmaceutical and therapeutic applications due to their unique properties. Both compounds are poised to contribute to sustainable and innovative advancements in various fields.
Read More:
Contents
- Differences Between Alcohols and Phenols
- Structural Makeup: The Hydroxyl Connection
- Physical Properties: A Glimpse into Molecular Behavior
- Chemical Reactivity: Unveiling Reaction Pathways
- Applications: Where Chemistry Meets Practicality
- Toxicity and Safety Considerations: Navigating Potential Risks
- Environmental Impact: Navigating Sustainability
- Biological Significance: Health and Medicinal Applications
- Future Trends: Innovations and Discoveries
- Alcohols or Phenols: Which One is Right for You?
- FAQs