| Aspect | Elements | Atoms |
|---|---|---|
| Definition | Pure substances composed of atoms with the same number of protons in their nuclei | The smallest units of matter, consisting of a nucleus (protons and neutrons) orbited by electrons |
| Composition | Combinations of identical atoms | Building blocks of elements; composed of protons, neutrons, and electrons |
| Representation | Represented by chemical symbols (e.g., H for hydrogen) | Represented by atomic symbols (e.g., 12(6)C for carbon-12) |
| Behavior | Exhibit unique physical and chemical properties | Follow the laws of physics and chemistry; participate in reactions and bonding |
| Variability | Over 118 elements with distinct properties | Exist as different isotopes, allotropes, and ions |
| Natural Abundance | Occur naturally in varying abundances | Can also be artificially synthesized in laboratories |
| Role in Reactions | Participate in chemical reactions, combining to form compounds | Undergo rearrangements in chemical reactions; do not change elemental identity |
| Atomic Structure | Composed of atoms with specific electron configurations | Consist of a nucleus (protons and neutrons) surrounded by electron energy levels |
| Charge | Neutral; have an equal number of protons and electrons | Can be charged; form ions by gaining or losing electrons |
| Mass Distribution | Mass distributed among protons, neutrons, and electrons | Mass concentrated in the nucleus; electrons contribute minimally to mass |
| Nuclear Transformations | Do not undergo nuclear transformations | Can undergo radioactive decay and transform into different elements |
| Quantum Behavior | Influence chemical behavior through electron configurations | Follow quantum mechanics, exhibiting wave-like behavior and uncertainty |
| Interactions in Bonds | Participate in chemical bonding based on electronegativity | Form bonds by sharing, gaining, or losing electrons |
| Unity in Laws | Diverse properties contribute to the periodic table | Obey the same laws of physics and chemistry, regardless of the element |
| Contribution to Matter | Fundamental units that combine to form compounds | Building blocks of elements; combine to create compounds and matter |
| Spatial Distribution | Exist as distinct substances | Occupy regions with varying probabilities due to wave-like behavior |
Picture the periodic table as a mosaic of nature’s alphabet, where each element represents a unique character in the grand narrative of matter. Elements, those elemental superheroes, are the heart and soul of this story. Composed of identical atoms, they exhibit a mesmerizing array of properties that shape our world. The periodic table showcases these characters like a colorful ensemble cast, with each element bringing its own set of traits to the stage.
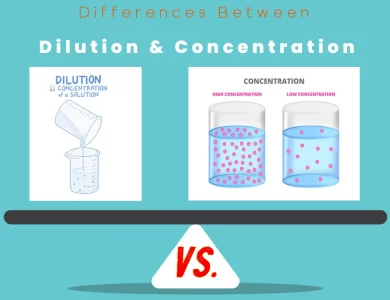
Differences Between Elements and Atoms
The main differences between elements and atoms lie in their fundamental nature and structure. Elements are pure substances composed of atoms sharing the same number of protons in their nuclei, each characterized by distinct properties. In contrast, atoms are the smallest units of matter, comprising a nucleus composed of protons and neutrons, surrounded by electrons. While elements represent various substances with unique traits, atoms are the intricate particles that constitute these elements, orchestrating their behavior and interactions.
The Basics
Elements: The Universe is an intricate tapestry woven from a mere 118 distinct elements. These elements are like nature’s alphabet, each with its unique properties and characteristics. An element is a pure substance composed of atoms that share the same number of protons in their atomic nuclei. This defining feature sets elements apart, giving them their own spots on the famed periodic table.
Atoms: Zoom in even further, and you’ll find atoms, the tiny constituents of elements. Atoms are the smallest indivisible units of matter, comprising a nucleus – housing protons and neutrons – orbited by electrons. These electrons dance in various energy levels, determining an atom’s chemical behavior. It’s like a miniature solar system where the nucleus is the sun and the electrons are planets.
Composition and Structure
Elements: Picture a box of crayons, each crayon representing an element. Elements consist of a single type of atom. For instance, the element hydrogen is an ensemble of atoms, each with one proton in its nucleus and one electron swirling around. Elements exhibit unique physical and chemical properties due to the specific arrangement of their atoms’ electrons.
Atoms: Imagine assembling a puzzle; atoms are the individual pieces. They come in various types, symbolizing different elements. An atom’s structure is elegantly organized. A central nucleus, packed with protons and neutrons, forms the core. The number of protons, also known as the atomic number, defines an atom’s elemental identity. Electrons whirl around the nucleus in energy levels or shells.
| Element | Atom |
|---|---|
| Composed of a single type of atom | Building blocks of elements |
| Represents a specific substance | Smallest unit of matter |
| Display distinctive properties | Structure: Nucleus (protons and neutrons) orbited by electrons |
Diversity in Nature
Elements: Nature is a grand showcase of these 118 elements. Some are as familiar as the oxygen we breathe, while others are as exotic as the rare earth metals. Each element has its story to tell, from hydrogen, the simplest and most abundant, to uranium, the heaviest naturally occurring element.
Atoms: The diversity continues at the atomic level. Atoms come in various shapes and sizes, determined by the arrangement of subatomic particles. Elements may have isotopes, which are atoms of the same element but with varying numbers of neutrons. Isotopes often have distinct properties, like carbon-12 and carbon-14, used in radiocarbon dating.
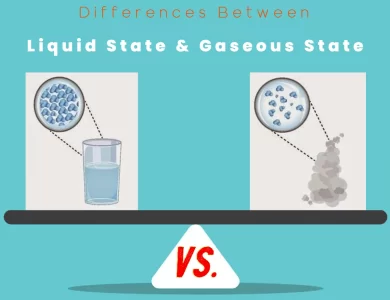
Unit of Matter vs. Fundamental Particle
Elements: Elements are the essential units of matter. Everything around us, from the towering mountains to the tiniest insects, is composed of these elemental building blocks. Each element contributes its unique characteristics to the matter it forms.
Atoms: Atoms, on the other hand, are like the fundamental particles of matter. They combine to create molecules, the intricate structures that make up substances. Molecules, in turn, weave the intricate fabric of materials in our world, shaping their properties.
Representation and Notation
Elements: Representing elements involves chemical symbols – one or two letters derived from the element’s name. These symbols are the shorthand way of denoting elements universally. For instance, ‘H’ stands for hydrogen, ‘O’ for oxygen, and ‘Au’ for gold (from the Latin “aurum”).
Atoms: Atoms are represented with atomic symbols, usually written in the format A(Z), where ‘A’ represents the mass number (protons + neutrons) and ‘Z’ is the atomic number (protons). For instance, carbon-12 is written as 12(6)C, signifying 12 as the mass number and 6 as the atomic number.
Interplay in Chemical Reactions
Elements: Chemical reactions are intricate dances where elements switch partners to create new compounds. These interactions are governed by the elements’ unique properties. For instance, the reaction between hydrogen and oxygen gives birth to the life-sustaining water molecule.
Atoms: Dive into the microscopic world of atoms within reactions. Here, atoms don’t change their elemental identities, but they rearrange into new configurations. The number and arrangement of electrons play a pivotal role in these transformations.
Building Matter: Complexity and Arrangement
Elements: Elements are the cornerstone of matter, each having its own distinct set of properties. Elements combine in various ratios to form compounds with diverse characteristics. For example, sodium (Na) and chlorine (Cl) join forces to create sodium chloride (NaCl), better known as table salt.
Atoms: Inside compounds, atoms come together in intricate ways. Their electron arrangements determine a compound’s behavior and properties. Take water (H2O) as an example: two hydrogen atoms bond with one oxygen atom, resulting in a substance with remarkable solvent properties and a wide range of uses.
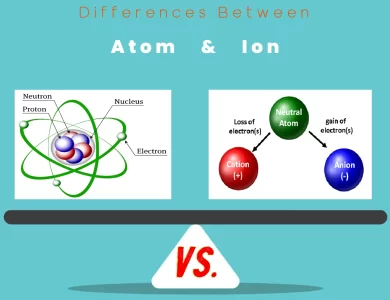
Mass vs. Charge
Elements: Elements possess mass, which can vary widely from one element to another. This mass is a result of the protons, neutrons, and electrons that constitute an atom. Elements are neutral, meaning they have an equal number of protons and electrons, resulting in no net electrical charge.
Atoms: Atoms also have mass due to their protons, neutrons, and electrons. However, their mass is concentrated in the nucleus. Unlike elements, atoms can be charged. An atom becomes an ion when it gains or loses electrons, leading to a net positive or negative charge, respectively.
The Quantum World: Electron Clouds and Energy Levels
Elements: Delving into the realm of elements, we encounter the captivating concept of electron configurations. Imagine electrons orbiting the nucleus in a dance of probability. These electron clouds define an element’s chemical behavior and reactivity. Elements in the same group of the periodic table share similar electron configurations, leading to comparable properties.
Atoms: Zooming further into the atomic structure, we discover the intricate world of energy levels. Electrons reside in these levels, each with a maximum capacity. The innermost level can hold up to 2 electrons, followed by 8 in the second, 18 in the third, and so on. This arrangement affects an atom’s stability and how it forms bonds with other atoms.
Radioactivity and Nuclear Transformations
Elements: Some elements possess an enigmatic quality – radioactivity. Elements like uranium undergo spontaneous nuclear transformations, emitting particles and energy. This phenomenon has practical applications, from medical imaging to energy generation.
Atoms: The heart of radioactivity lies within the nucleus of atoms. Unstable nuclei undergo decay, transforming into different elements. For instance, uranium-238 eventually decays into lead-206 through a series of radioactive transformations.
Natural Abundance and Artificial Synthesis
Elements: Nature has crafted an exquisite tapestry of elements, each with its own story. Some, like oxygen and carbon, are ubiquitous, while others, like gold and platinum, are rarer gems. These naturally occurring elements shape the Earth’s composition.
Atoms: Atoms can also be synthesized, adding a new layer to the atomic world. Scientists can create elements that don’t exist in nature by bombarding nuclei with particles. For example, element 118, oganesson (Og), was artificially synthesized in a laboratory.
Beyond the Basics: Isotopes and Allotropes
Elements: Elements are not always as simple as they seem. Enter isotopes – atoms of the same element with differing numbers of neutrons. Isotopes can have unique properties; for example, carbon-14 finds use in radiocarbon dating.
Atoms: Allotropes are another intriguing facet. Some elements can exist in different structural forms, each with distinct properties. Carbon, for instance, can be found as graphite, diamond, or even nanotubes.
The Puzzle of Subatomic Particles: Protons, Neutrons, and Electrons
Elements: At the core of each element lies a nucleus, a tight-knit cluster of protons and neutrons. This nucleus defines an element’s identity. The number of protons determines the element’s atomic number, while the sum of protons and neutrons gives the atomic mass.
Atoms: The symphony of particles continues within atoms. Protons, with their positive charge, huddle in the nucleus, balanced by neutral neutrons. Electrons, lightweight and negatively charged, elegantly orbit the nucleus in various energy levels.
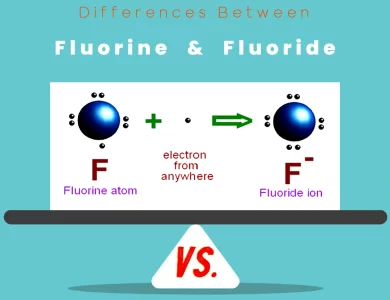
Electronegativity and Chemical Bonding
Elements: Electronegativity, a fundamental property of elements, plays a pivotal role in chemical bonding. It’s like the element’s magnetism for electrons. Elements with high electronegativity, like fluorine, readily attract electrons, forming ionic or covalent bonds.
Atoms: The electronegativity dance extends to atoms within compounds. Atoms tug and share electrons to achieve stable configurations. This tug-of-war creates the myriad molecules that compose our surroundings.
From Elements to Compounds: The Alchemical Transformation
Elements: Elements are the prima materia, the raw materials of chemistry’s alchemy. By combining elements, we fashion compounds – substances with distinct properties. The elemental ballet of combination leads to an array of compounds, from the simplicity of water to the complexity of DNA.
Atoms: Atoms become master performers in this elemental dance, arranging themselves in precise configurations to create compounds. The arrangement of atoms and the types of bonds they form dictate a compound’s behavior and function.
The Quantum Puzzle: Wave Functions and Uncertainty
Elements: Quantum mechanics whispers its mysteries to elements. Each electron’s path is described by a wave function, a mathematical expression that predicts its probable location. The Heisenberg uncertainty principle adds an enigmatic twist – we can’t know both an electron’s position and momentum with absolute certainty.
Atoms: Dive into atoms’ quantum world, and wave functions guide electrons’ behavior. Electrons don’t follow predictable paths; instead, they occupy regions with varying probabilities. This uncertainty underpins the delicate dance of chemical reactions.
The Unified Symphony of Chemistry
Elements: The elements’ diverse array forms the periodic table, a symphony of properties and patterns. Elements are the musicians, each playing a unique note in this chemical orchestra. The table organizes elements based on their properties, revealing trends and relationships.
Atoms: The unity of atoms binds this symphony. No matter the element, atoms obey the same laws of physics and chemistry. Electrons don’t discriminate between elements; they follow the same principles, orchestrating reactions and transformations.
FAQs
Elements are pure substances composed of atoms with the same number of protons in their nuclei, each displaying unique properties. Atoms, on the other hand, are the smallest units of matter, consisting of a nucleus composed of protons and neutrons, orbited by electrons.
Elements are made up of identical atoms that share common properties. Atoms, however, are the building blocks of elements, comprised of protons, neutrons, and electrons, each contributing to their distinct characteristics.
Elements play a crucial role in chemical reactions as they combine to form compounds with varying properties. Atoms, within these compounds, rearrange and share electrons, driving the transformations and interactions observed in reactions.
The periodic table showcases elements as a diverse ensemble, each occupying a unique place based on its properties. Atoms, constituting these elements, define their positions and determine their chemical behaviors within the table’s organized structure.
No, atoms maintain their elemental identity in reactions. While they may rearrange and form new compounds, their atomic structure and elemental properties remain constant.
Electron configuration, the arrangement of electrons in energy levels, influences the chemical behavior of elements. At the atomic level, electron configuration determines an atom’s stability and its role in bonding with other atoms.
Isotopes are atoms of the same element with differing numbers of neutrons. Elements can have multiple isotopes, which contribute to variations in their atomic mass while maintaining the same elemental identity.
Elements form the basis of the periodic table, with each element represented by its unique properties. Atoms are the constituents of these elements, dictating their placement and behavior within the organized framework of the periodic table.
Elements combine in various ratios to create compounds with a wide range of properties. Atoms, through their configurations and interactions, determine the behavior and characteristics of these compounds, thereby contributing to the diversity of matter observed in the world around us.
Read More:
Contents
- Differences Between Elements and Atoms
- The Basics
- Composition and Structure
- Diversity in Nature
- Unit of Matter vs. Fundamental Particle
- Representation and Notation
- Interplay in Chemical Reactions
- Building Matter: Complexity and Arrangement
- Mass vs. Charge
- The Quantum World: Electron Clouds and Energy Levels
- Radioactivity and Nuclear Transformations
- Natural Abundance and Artificial Synthesis
- Beyond the Basics: Isotopes and Allotropes
- The Puzzle of Subatomic Particles: Protons, Neutrons, and Electrons
- Electronegativity and Chemical Bonding
- From Elements to Compounds: The Alchemical Transformation
- The Quantum Puzzle: Wave Functions and Uncertainty
- The Unified Symphony of Chemistry
- FAQs