Science
Prepare to embark on an enlightening journey as we uncover the nuances and distinctions that make the scientific field so diverse and awe-inspiring. Whether you’re a science enthusiast or a curious mind seeking knowledge, this collection of content will satiate your thirst for understanding the intricacies of scientific terminology and concepts.
-

pOH vs pH
In the realm of chemistry, pH and pOH stand as vital parameters that decode the intricate world of acidity and alkalinity within solutions. These two concepts, often intertwined, possess distinct focuses that offer complementary insights into the nature of a solution. pH represents the potential of hydrogen ions in a solution. Operating on a logarithmic scale from 0 to 14, it indicates the concentration of hydrogen ions – lower pH values signal higher acidity, while higher values reflect increased alkalinity. With the formula pH = -log[H⁺], this measure intricately balances the concentration of hydrogen ions, playing a pivotal role in various scientific domains. pOH, less frequently discussed but equally significant, spotlights hydroxide ions' concentration in a solution. Much like pH, pOH spans from 0 to 14 on a logarithmic scale. As pOH values decrease, the solution's basicity intensifies, demonstrating its rich alkaline properties. Unveiling the contrasting intricacies of pH and pOH is akin to peering through distinct windows into the chemical landscape. While pH takes center stage in numerous applications, pOH's role in revealing the behavior of basic solutions is no less important. Together, these parameters empower chemists, scientists, and learners to understand and interpret the language of solutions – a cornerstone in unraveling the mysteries of the natural world.
-

pKb vs pKa
Delving into the realm of acid-base equilibrium, pKa and pKb emerge as crucial concepts for understanding the strengths of acids and bases, respectively. The disparity between these two measures lies at the heart of their functionalities. pKa stands as the negative logarithm of the acid dissociation constant (Ka), indicating the tendency of an acid to donate protons. In contrast, pKb embodies the negative logarithm of the base dissociation constant (Kb), illustrating the basicity of a base by its propensity to accept protons. The relationship between pKa and pKb unveils an intriguing dance of equilibrium. As pKa decreases, acid strength amplifies; as pKb decreases, base strength intensifies. The Henderson-Hasselbalch equation, an essential tool, elucidates the pH relation in solutions involving these measures. These concepts materialize in various applications, from titrations and buffering systems to their profound impact on biochemical processes and the environment. The tables presented herein encapsulate the multifaceted dissimilarities between pKa and pKb, covering their definitions, calculations, and implications. As you navigate the intricacies of chemistry, the understanding of pKa and pKb will undoubtedly serve as your guiding compass, aiding you in deciphering the nuanced symphony of acids and bases that shapes the chemical world.
-

Titration vs Back Titration
In the captivating world of chemistry, two intriguing techniques, titration and back titration, take the spotlight. These methods, while both used for quantitative analysis, differ in their approaches and applications. Titration involves the gradual addition of a known solution to an unknown solution until a reaction reaches completion, allowing the determination of the unknown's concentration. It's a direct method, ideal for reactions with clear endpoints and solutions of known concentrations. The precision lies in its simplicity, offering reliable results in routine analyses. On the other hand, back titration takes a more indirect route. Here, the unknown solution reacts with an excess of a known reagent, with the leftover excess later titrated to determine how much reacted initially. This approach is strategic for complex reactions or when the endpoint is challenging to detect. While it sacrifices immediate precision for accuracy, it navigates intricate scenarios that direct titration might stumble upon. The choice between these techniques depends on the nature of the analysis. Titration is a swift arrow in the quiver for straightforward cases, while back titration shines in tackling the enigmatic. Each approach reveals hidden insights, showcasing the artistry and precision that define the world of analytical chemistry.
-

Speed vs Pace
In the realm of athletic pursuits, the contrast between pace and speed is more than meets the eye. Like two sides of a coin, these terms often intertwine, yet their roles are uniquely defined. Pace strides in as the consistent heartbeat of endurance, measuring the time taken per unit of distance covered. It's your steady companion during marathons, ensuring you don't burn out too soon, maintaining a rhythm that leads you triumphantly to the finish line. Think of it as the compass guiding your journey through long stretches. Speed, on the other hand, takes the spotlight when urgency and rapidity are at play. It's not concerned with distance; rather, it's all about the rate of motion, the swift propulsion that sends you hurtling toward victory in a sprint. Whether it's a 100-meter dash or a quick burst of acceleration in sports like tennis or basketball, speed is your ally in the pursuit of lightning-fast accomplishments. To measure pace, think "minutes per mile" or "minutes per kilometer." It's the art of maintaining a steady rhythm over a prolonged journey. Speed, on the other hand, revels in metrics like "miles per hour" or "meters per second," slicing through the essence of time itself, emphasizing the sheer rapidity of motion. So, next time you're pounding the pavement or sprinting on the track, remember that pace and speed, though harmonious, dance to different tunes. They're the yin and yang of your athletic endeavors, offering diverse ways to conquer challenges, set records, and embrace the full spectrum of motion.
-

Zinc vs Zinc Oxide
Embarking on a journey into the world of materials, we delve into the intriguing disparities between zinc and zinc oxide. While they share a common elemental basis, their unique attributes shape their roles in various industries and applications. Zinc, a versatile transition metal, boasts malleability and ductility, making it an ideal candidate for galvanization and alloy production. Its essential micronutrient status in biological systems harmonizes with its potential toxicity when consumed in excess. Environmental considerations highlight the importance of proper disposal to prevent harm to ecosystems. On the other hand, zinc oxide, a compound of zinc and oxygen, dons the form of a white powder with a higher melting point. Its properties, including UV-absorption and mild antiseptic effects, drive its applications in sunscreens, cosmetics, and pharmaceuticals. The safety aspect of zinc oxide is underscored by its Generally Recognized as Safe (GRAS) status, contrasting with zinc's careful dietary moderation. As we venture into their applications, manufacturing methods, and solubility behaviors, a comprehensive understanding of these materials emerges. While zinc finds its place in protective coatings and semiconducting materials, zinc oxide takes center stage in modern technologies, nanoscience, and skincare. In this exploration of zinc vs zinc oxide, we navigate their differences, unveiling the multifaceted nature of materials science and the intricate roles these substances play in shaping our world.
-

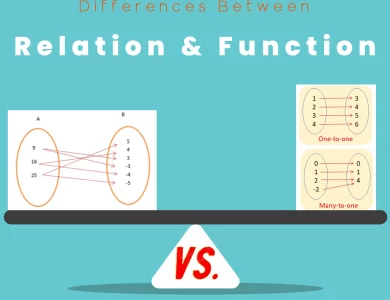
Function vs Relation
Delving into the intricate world of mathematics, it becomes evident that relations and functions stand as cornerstones of numerical analysis. While their surface might hint at similarities, a deeper examination reveals stark differences that define their roles in mathematical contexts. Relations act as bridges connecting elements from distinct sets through ordered pairs. Akin to social connections, they display the interplay between variables without enforcing a strict one-to-one mapping. From graphs to tables, relations come alive through various representations, capturing the multifaceted nature of connections. Functions, on the other hand, introduce a layer of predictability. These specialized relations establish a one-to-one connection, where each input corresponds to a unique output. Often portrayed through equations or mappings, functions guide calculations and predictions, transforming abstract ideas into practical applications. In real-world scenarios, relations mirror social networks, representing diverse connections among entities. In contrast, functions assume roles as predictive tools, estimating outcomes based on given inputs. Their distinct attributes have far-reaching implications, shaping the way we model relationships and forecast results in fields ranging from physics to economics. The journey through relations and functions uncovers the beauty of mathematical diversity. Just as the intricacies of relations weave a complex web of connections, the structured elegance of functions provides a roadmap for predicting and understanding the world around us. As we embrace these differences, we enrich our mathematical toolkit, equipping ourselves to tackle a wide array of challenges with precision and insight.
-

Salt (Sodium Chloride) vs MSG (Monosodium Glutamate)
Embarking on a culinary journey means embracing a world of flavors and textures. In the realm of taste transformation, two essential ingredients, MSG and salt, hold distinct roles that shape the art of cooking. Monosodium Glutamate (MSG), often misunderstood due to myths, is a flavor enhancer that elevates specific tastes, particularly the umami sensation. This compound, composed of sodium and glutamate, doesn't alter textures directly but crafts a harmonious symphony of flavors. On the other hand, salt, the ubiquitous ingredient, enhances overall taste perception while playing a crucial role in altering textures. Its sodium and chlorine composition infuses dishes with the much-loved saltiness that tantalizes our palates. From a health perspective, moderation is essential when using both MSG and salt. Scientific research supports the safety of MSG in moderate quantities, debunking past misconceptions. In contrast, excessive salt intake has been linked to health issues such as high blood pressure. Understanding their distinctive culinary applications is key. While MSG shines in umami-rich dishes, salt's prowess is evident in various cuisines, both sweet and savory. In the modern culinary landscape, MSG's complex enhancement of flavors pairs with salt's multifaceted contributions, resulting in dishes that captivate not only the taste buds but also the imagination. A pinch of knowledge about these two ingredients allows culinary enthusiasts to master the art of seasoning, transforming everyday meals into gastronomic delights.
-

Non Parametric vs Parametric
Delving into the world of statistical analysis, one encounters the pivotal choice between parametric and non-parametric methods. These two methodologies anchor diverse statistical explorations, each boasting distinct strengths and limitations. Parametric methods, resembling precision instruments, make assumptions about the data's distribution. They thrive when data adheres to the assumed distribution, yielding accurate results. However, these methods stumble when data strays from the expected path, making assumptions a potential Achilles' heel. They demand larger sample sizes for robust outcomes and can be sensitive to outliers. Non-parametric methods, on the other hand, showcase flexibility akin to chameleons. They operate without specific distribution assumptions, making them versatile in varied data scenarios. They excel in situations where data distribution remains an enigma, when assumptions crumble, or when small sample sizes are at play. Their robustness against outliers offers a safety net in turbulent data landscapes. While parametric methods excel in cases where assumptions are valid, non-parametric methods offer a haven for situations shrouded in uncertainty. The choice between these methodologies hinges on data characteristics, research goals, and the balance between accuracy and flexibility. As technology evolves, hybrid approaches may bridge the gap, leveraging both paradigms to harness the power of precision and adaptability. Armed with this understanding, statisticians navigate the analytical cosmos with confidence, ensuring the chosen method aligns seamlessly with data realities and research objectives.
-

Amphibian vs Reptile
Embark on a fascinating journey through the captivating realms of reptiles and amphibians as we unravel the intriguing differences that set these two remarkable groups apart. From their distinct skin characteristics to their unique reproductive strategies, the divergence between reptiles and amphibians is a captivating tale of adaptation and evolution. Reptiles, characterized by scaled skin made of keratin, wield these scales as armor, providing protection while aiding in the art of camouflage. On the other hand, amphibians flaunt moist and permeable skin, serving multiple purposes—respiration, defense, and even communication—within their ever-changing environments. Diving deeper, we encounter their contrasting approaches to reproduction. Reptiles lay eggs enveloped in protective shells, with incubation methods ranging from nesting to ovoviviparity. In contrast, amphibians lay exposed eggs that rely heavily on moisture for proper development, and many undergo remarkable metamorphoses as they transition from aquatic larvae to terrestrial adults. The tale continues with their habitat preferences—reptiles' dominance on land and their reliance on external heat sources for thermoregulation, and amphibians' duality in thriving both in water and on land, thanks to their permeable skin.
-

Reptile vs Mammal
Delving into the captivating realm of the natural world, we uncover the intriguing contrasts that set mammals and reptiles apart. Mammals, characterized by their warm-blooded nature and fur-covered bodies, stand in stark contrast to the resilient reptiles with their scaly skin and cold-blooded physiology. Reproduction unfolds differently in these two groups, with mammals giving birth to live young and providing nurturing care, while reptiles often lay eggs or give birth to independent offspring. From communication strategies to habitats and evolutionary histories, each facet of these creatures unveils a tale of survival and adaptation. Mammals communicate through complex vocalizations and flourish in various environments due to their internal temperature regulation. Reptiles, on the other hand, utilize visual cues and thrive in diverse ecosystems thanks to their ability to adapt to fluctuating temperatures. As we delve into these differences, we gain a deeper appreciation for the intricate diversity that enriches our natural world. Join us on this captivating journey as we explore the marvels of Mammal vs Reptile distinctions, uncovering the threads that weave the fabric of life on Earth.