| Aspect | pH | pOH |
|---|---|---|
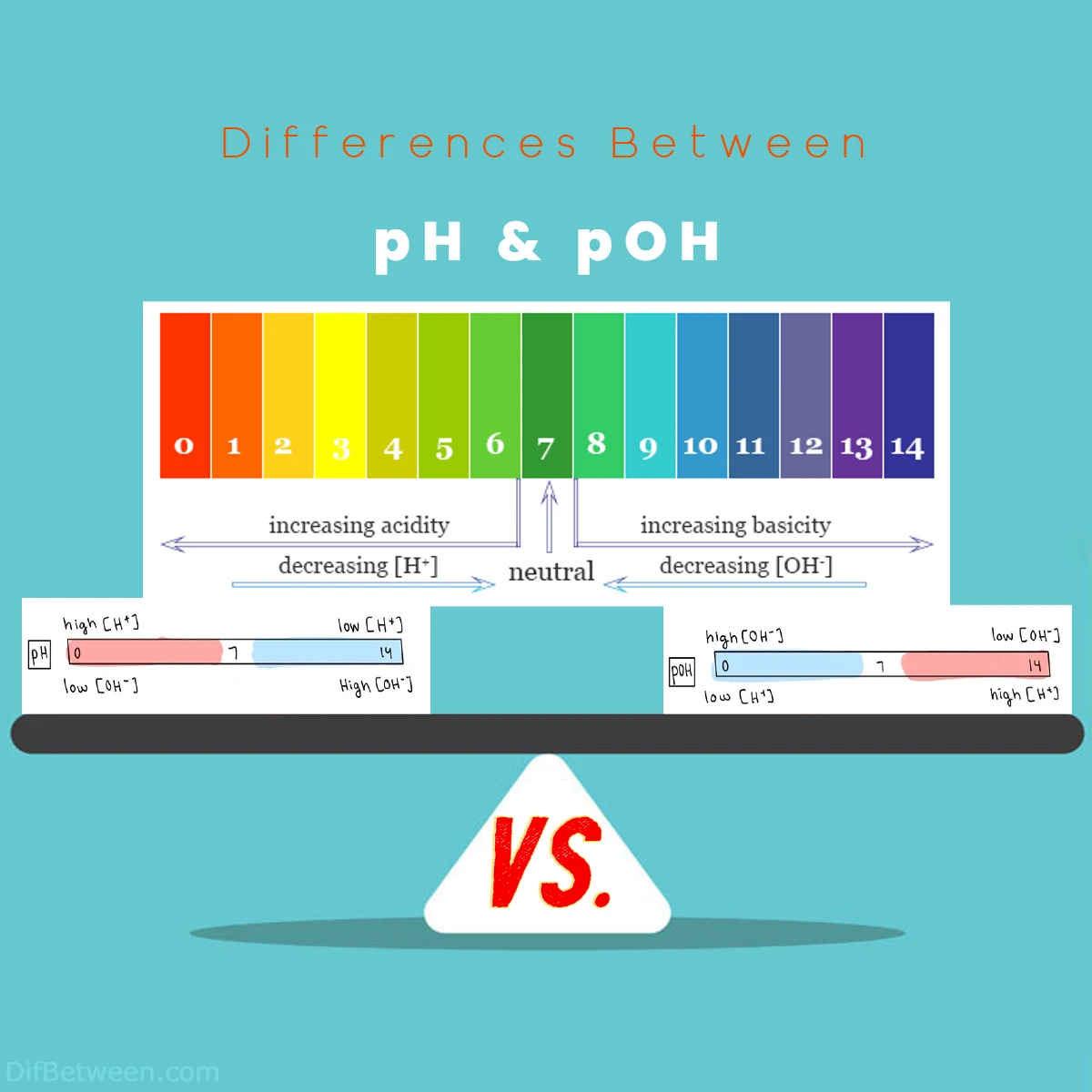
| Definition | pH is a measure of hydrogen ion (H⁺) concentration in a solution. It indicates the acidity or alkalinity of a solution. | pOH is a measure of hydroxide ion (OH⁻) concentration in a solution. It provides insights into the basicity or acidity of a solution. |
| Calculation Formula | pH = -log[H⁺] | pOH = -log[OH⁻] |
| Measurement Range | pH values range from 0 (highly acidic) to 14 (highly alkaline). | pOH values also range from 0 (highly basic) to 14 (highly acidic). |
| Neutral Value | pH 7 represents neutrality (equal concentrations of H⁺ and OH⁻ ions). | pOH 7 also represents neutrality. |
| Logarithmic Scale | pH operates on a logarithmic scale, with each pH unit representing a tenfold difference in H⁺ concentration. | pOH, like pH, operates on a logarithmic scale, with each unit representing a tenfold difference in OH⁻ concentration. |
| Relationship | pH and pOH are inversely related. As pH increases, pOH decreases, and vice versa. | pH and pOH are inversely related. When one increases, the other decreases to maintain equilibrium. |
| Acidic Solutions | Lower pH values indicate higher H⁺ concentration, resulting in stronger acidity. | Higher pOH values indicate lower OH⁻ concentration, making the solution more acidic. |
| Basic Solutions | Higher pH values indicate lower H⁺ concentration, leading to higher alkalinity. | Lower pOH values indicate higher OH⁻ concentration, indicating stronger basicity. |
| Equilibrium System | pH measures the concentration of H⁺ ions in relation to H₂O autoionization. | pOH measures the concentration of OH⁻ ions in relation to H₂O autoionization. |
| Measurement Tools | pH can be measured using pH indicators or electronic pH meters. | pOH is often calculated from OH⁻ concentration using the negative logarithm formula. |
| Application Range | pH measurements are commonly used in various fields, including biology, chemistry, and environmental science. | pOH measurements are less commonly discussed but are crucial in understanding basic solutions’ behavior. |
These two concepts might sound like a tongue-twister at first, but fear not – by the end of this narrative, you’ll be equipped with a crystal-clear understanding of the key differences between pH and pOH.
Differences Between pH and pOH
The primary differences between pH and pOH lie in their distinct measurement focuses within the realm of chemistry. pH quantifies the concentration of hydrogen ions (H⁺) in a solution, portraying its acidity or alkalinity. On the other hand, pOH measures the concentration of hydroxide ions (OH⁻) in a solution, offering insights into its basicity or acidity. While both pH and pOH scales range from 0 to 14, their inverse relationship sets them apart – as pH values rise, pOH values decrease, and vice versa. This intrinsic connection arises from the equilibrium between hydrogen and hydroxide ions in solutions. In essence, pH and pOH together paint a comprehensive picture of a solution’s chemical nature, illuminating the dynamic interplay between acidity and basicity.
pH and pOH: The Fundamentals
Let’s kick off this exploration by delving into the basics. pH and pOH are both measurements that provide insights into the acidity or alkalinity of a solution. They are fundamental indicators used by chemists to quantify the concentration of hydrogen ions (H⁺) and hydroxide ions (OH⁻) in a solution, respectively. These ions play a pivotal role in determining the nature of a solution – whether it’s acidic, neutral, or alkaline (basic).
The pH Perspective
pH: The Measure of Hydrogen Ion Concentration
pH, or “potential of hydrogen,” is a metric used to express the concentration of hydrogen ions in a solution. It operates on a logarithmic scale, which might sound intimidating, but it’s actually quite straightforward. The pH scale ranges from 0 to 14, where 0 is highly acidic, 7 is neutral, and 14 is highly alkaline. Each unit on the pH scale represents a tenfold difference in the concentration of hydrogen ions.
Picture it like a seesaw – as the pH value decreases, the acidity of the solution increases, signifying a higher concentration of hydrogen ions. Conversely, as the pH value rises, the solution becomes more alkaline due to a higher concentration of hydroxide ions and a lower concentration of hydrogen ions.
Now, let’s dive into the world of pOH!
The pOH Perspective
pOH: Decoding Hydroxide Ion Concentration
Enter pOH, a less commonly discussed yet equally significant parameter in the realm of chemistry. While pH deals with hydrogen ions, pOH focuses on hydroxide ions. Just as the pH scale ranges from 0 to 14, the pOH scale also spans from 0 to 14, with 7 representing neutrality.
Calculating pOH is a breeze. It’s the negative logarithm of the hydroxide ion concentration. So, as the pOH value decreases, the alkalinity of the solution increases, indicating higher hydroxide ion concentration.
Head-to-Head: pH vs. pOH
Measurement Focus: Hydrogen Ions vs. Hydroxide Ions
The most apparent distinction between pH and pOH lies in their measurement focus. pH centers around the concentration of hydrogen ions, while pOH zeroes in on the concentration of hydroxide ions. It’s like comparing apples to oranges – both are fruit, but each brings a unique flavor to the table.
Calculation Method: Positive vs. Negative Logarithm
Another illuminating contrast is how these values are calculated. pH involves taking the negative logarithm of the hydrogen ion concentration. The formula for pH is as follows:
pH=−log[H+]pH=−log[H+]
On the other hand, pOH is determined by taking the negative logarithm of the hydroxide ion concentration, following this formula:
pOH=−log[OH−]pOH=−log[OH−]
pH and pOH Relationship: Dynamic Duo
pH and pOH are like two sides of the same coin, and there’s an elegant relationship that binds them together. This relationship stems from the autoionization of water, where water molecules simultaneously act as acids and bases. This equilibrium leads to the formation of equal concentrations of hydrogen ions (H+H+) and hydroxide ions (OH−OH−) in a neutral solution. As a result, a change in pH directly influences pOH and vice versa.
The pH-pOH Conversion Table: Bridging the Gap
For those who thrive on data visualization, a pH-pOH conversion table can be your trusty guide. This table showcases the interplay between pH and pOH values for various types of solutions, from strongly acidic to strongly alkaline. It’s a bridge that allows you to traverse between the realms of hydrogen and hydroxide ions effortlessly.
Here’s a snippet of what the conversion table looks like:
| pH Value | pOH Value | Acidic/Alkaline Nature |
|---|---|---|
| 0 | 14 | Strongly Acidic |
| 1 | 13 | |
| 2 | 12 | |
| 3 | 11 | |
| 4 | 10 | |
| 5 | 9 | |
| 6 | 8 | |
| 7 | 7 | Neutral |
| 8 | 6 | |
| 9 | 5 | |
| 10 | 4 | |
| 11 | 3 | |
| 12 | 2 | |
| 13 | 1 | |
| 14 | 0 | Strongly Alkaline |
pH and pOH in Action: Real-World Applications
These seemingly abstract concepts have real-world implications that stretch across various disciplines. From biology and medicine to environmental science and industry, pH and pOH are the unsung heroes that shape our understanding and interactions with the world.
Biology and Medicine: The Goldilocks Principle
In the realm of biology and medicine, maintaining the right pH and pOH levels is crucial for the proper functioning of biological systems. Enzymes, the molecular workhorses that drive biochemical reactions in our bodies, exhibit optimal activity within specific pH and pOH ranges. Deviations from these ranges can lead to enzyme denaturation and the disruption of essential physiological processes.
For instance, the pH level of our blood is tightly regulated around 7.4, slightly alkaline. This balance ensures that our cells can carry out vital functions effectively. The pH-pOH duo also plays a role in medication formulation. Certain drugs work optimally when administered in specific pH environments.
Environmental Science: Aquatic Ecosystems’ Silent Dance
In aquatic ecosystems, pH and pOH play a pivotal role in determining the health and viability of various life forms. The pH of water bodies influences the solubility of minerals and nutrients, affecting the growth and survival of aquatic plants and organisms. Acid rain, a consequence of human activities releasing sulfur dioxide and nitrogen oxides into the atmosphere, can drastically lower the pH of water bodies, wreaking havoc on aquatic ecosystems.
Industry and Agriculture: From Factories to Farms
In the industrial landscape, pH and pOH measurements guide processes across a spectrum of applications. From wastewater treatment plants to food and beverage manufacturing, maintaining specific pH and pOH conditions is essential to ensure efficient processes and product quality.
In agriculture, the pH of soil directly impacts nutrient availability to plants. Different crops thrive within specific pH ranges, and farmers often adjust soil pH to maximize yield and quality.
The pH-pOH Conundrum: When Things Get Tricky
As with any concept, there are instances when the pH-pOH duo might leave you scratching your head. One common confusion arises when interpreting a solution’s pH or pOH value. An acidic solution doesn’t necessarily mean it’s devoid of hydroxide ions, and a basic solution doesn’t lack hydrogen ions.
Consider this: A solution with a pH of 2 is highly acidic, but it still contains a minuscule concentration of hydroxide ions. Similarly, a solution with a pOH of 4 is basic, though it contains a trace of hydrogen ions.
The Equilibrium Game: pH and pOH Shifts
Now, let’s unravel the captivating equilibrium game that pH and pOH engage in. When dealing with solutions, the concentrations of hydrogen ions and hydroxide ions are inversely related. This means that as one ion’s concentration increases, the other’s decreases to maintain a delicate balance.
Imagine you have a solution with a pH of 3. This tells you that the concentration of hydrogen ions is relatively high. But what about the concentration of hydroxide ions? Well, it’s lower, keeping the equilibrium intact. As a result, the pOH of the solution is less than 11 (pOH = 14 – pH). This interplay showcases the dynamic relationship between pH and pOH – as one value rises, the other falls.
The Specter of Neutral Solutions: A pH-pOH Tug of War
In the realm of neutral solutions, where the pH is 7, the pH-pOH tug of war reaches its peak. With equal concentrations of hydrogen and hydroxide ions, the pH and pOH values are both 7. This equilibrium symbolizes a perfect balance between acidity and alkalinity, resulting in a neutral solution that’s neither too sour nor too bitter.
But wait – what happens when a solution ventures into the realms of extreme acidity or intense alkalinity?
Extreme pH-pOH: Breaking the Boundaries
Picture a solution with a pH of 0 – a scenario of intense acidity. In this case, the concentration of hydrogen ions is astoundingly high. As a consequence, the concentration of hydroxide ions must be almost negligible to maintain equilibrium. Calculating the pOH for this solution gives us a value of 14 (pOH = 14 – pH), indicating an extreme absence of hydroxide ions.
Conversely, envision a solution with a pH of 14 – a scenario of remarkable alkalinity. Here, the concentration of hydroxide ions is remarkably high, causing the concentration of hydrogen ions to be extremely low. Calculating the pOH gives us a value of 0 (pOH = 14 – pH), signifying an exceptional dearth of hydrogen ions.
pH and pOH in Solvent Wonderland: Aqueous and Beyond
The realm of pH and pOH isn’t limited to just aqueous solutions. These concepts stretch their boundaries to non-aqueous solvents, showcasing their versatile nature. However, it’s essential to note that the pH scale is primarily tailored for water-based solutions. When delving into non-aqueous environments, certain adjustments might be needed to ensure accurate pH and pOH measurements.
pH and pOH Measurement: The Tools of the Trade
Armed with the knowledge of pH and pOH, you might wonder how scientists and chemists measure these elusive parameters. The answer lies in a range of potent tools that decipher the pH and pOH puzzle.
pH Measurement: pH Indicators and pH Meters
pH indicators are compounds that exhibit distinct color changes across different pH ranges. These visual cues allow researchers to estimate the approximate pH of a solution. For more precise measurements, pH meters come to the rescue. These electronic devices offer accurate pH readings by measuring the voltage generated when a pH-sensitive electrode comes into contact with the solution.
pOH Measurement: Calculations and Indicators
When it comes to pOH measurements, it’s a bit more hands-on. Chemists often calculate pOH using the negative logarithm formula. Additionally, certain indicators can change color at specific pOH values, aiding in visual assessments of a solution’s pOH level.
The pH-pOH Relationship Revisited: A Harmonious Symphony
Remember the harmony between pH and pOH, stemming from the autoionization of water? This equilibrium forms a symphony that resonates through the world of chemistry. Changes in one parameter lead to proportional shifts in the other, ensuring that the equilibrium remains unbroken.
When an acidic substance is added to a solution, it donates hydrogen ions, causing the pH to decrease. However, this addition also triggers the consumption of hydroxide ions, thus increasing the pOH. Similarly, adding a basic substance triggers the opposite dance – an increase in pH and a decrease in pOH.
pH-pOH Duo: Partners in Titration
The pH-pOH partnership shines especially bright in the realm of titration, a powerful analytical technique. During titration, a solution with a known concentration is gradually added to another solution of unknown concentration until the reaction between the two is complete. This point is called the equivalence point.
In acid-base titrations, the pH-pOH relationship takes center stage. As you approach the equivalence point, the pH of the solution changes dramatically, indicating the completion of the reaction. The pOH, too, shows a corresponding shift, but in the opposite direction.
pH and pOH: Our pHinal Thoughts
As we draw the curtain on this exploration of pH and pOH, we’ve journeyed through the vibrant tapestry of chemistry. These two parameters, pH and pOH, are the threads that weave the story of acidity and alkalinity in the solutions that surround us.
From their distinct measurement focuses to their symbiotic relationship, pH and pOH offer us a glimpse into the dynamic world of chemical equilibrium. They help us understand not only the behavior of solutions but also their impact on biology, the environment, industry, and beyond.
So, whether you’re a chemistry enthusiast, a student venturing into the realms of science, or simply a curious soul seeking answers to the mysteries of the universe, remember that the tale of pH and pOH holds the key to deciphering the language of solutions and their hidden stories.
FAQs
pH measures the concentration of hydrogen ions (H⁺) in a solution, indicating its acidity or alkalinity. pOH measures the concentration of hydroxide ions (OH⁻) in a solution, revealing its basicity or acidity.
pH is calculated using the formula pH = -log[H⁺], where [H⁺] represents the concentration of hydrogen ions. pOH is calculated using the formula pOH = -log[OH⁻], where [OH⁻] represents the concentration of hydroxide ions.
Yes, both pH and pOH scales range from 0 to 14. A pH of 7 is neutral, while values below 7 are acidic, and values above 7 are alkaline. Similarly, a pOH of 7 is neutral, but values below 7 are basic, and values above 7 are acidic.
pH and pOH are inversely related. As pH increases, pOH decreases, and vice versa. This relationship arises from the autoionization of water, where changes in one ion’s concentration affect the other to maintain equilibrium.
pH values are essential in various applications, including medicine, biology, and industry, to understand the acidic or alkaline nature of solutions. pOH values are less commonly discussed but provide insights into basic solutions, particularly in analytical chemistry.
Absolutely. In biology, pH affects enzyme activity and bodily functions. In the environment, pH influences aquatic ecosystems’ health. In industry, pH control is crucial for various processes, from manufacturing to wastewater treatment.
While pH and pOH are tailored for aqueous solutions, they can be adapted for non-aqueous solvents. However, adjustments may be necessary to account for different ion behaviors in these environments.
pH can be measured using pH indicators, which change color at different pH levels, or electronic pH meters for accurate readings. pOH is often calculated using the negative logarithm formula and might not have as many measurement tools dedicated to it.
Both pH and pOH are important in their own right. pH is more commonly discussed due to its broader relevance, but pOH provides insights into the less-explored world of basic solutions, completing the understanding of a solution’s full spectrum.
Yes, pH and pOH can change simultaneously in a solution. When external factors alter the concentration of either hydrogen ions or hydroxide ions, both pH and pOH will shift to maintain equilibrium between the two ions.
Read More:
Contents
- Differences Between pH and pOH
- pH and pOH: The Fundamentals
- Head-to-Head: pH vs. pOH
- pH and pOH Relationship: Dynamic Duo
- The pH-pOH Conversion Table: Bridging the Gap
- pH and pOH in Action: Real-World Applications
- The pH-pOH Conundrum: When Things Get Tricky
- Extreme pH-pOH: Breaking the Boundaries
- pH and pOH in Solvent Wonderland: Aqueous and Beyond
- pH and pOH Measurement: The Tools of the Trade
- pH-pOH Duo: Partners in Titration
- pH and pOH: Our pHinal Thoughts
- FAQs






