| Aspect | Phenol | Phenyl |
|---|---|---|
| Definition | Compound with hydroxyl group (-OH) attached to benzene ring | Functional group derived from benzene by removing one hydrogen atom |
| Chemical Formula | C6H6O | C6H5 |
| Structure | Benzene ring with hydroxyl group (-OH) | Part of benzene ring without functional groups |
| Functional Group | Hydroxyl (-OH) group attached to benzene ring | Benzene ring with one hydrogen atom removed |
| Physical State | Typically a colorless crystalline solid | Not a standalone compound, but a structural fragment |
| Solubility | Soluble to some extent in water due to hydrogen bonding | Insoluble in water; nonpolar |
| Acidity | Weak acid due to the hydroxyl group | Not acidic |
| Reactivity | Participates in electrophilic substitution, hydrogen bonding, and other reactions | Indirectly influences reactivity of compounds it’s part of |
| Toxicity | Caustic and toxic, especially in concentrated forms | Generally not toxic on its own, but safety considerations apply |
| Applications | Industrial chemicals, medical disinfectants, pharmaceutical precursor | Drug design, agrochemical enhancement, structural modification |
| Industrial Uses | Production of plastics, detergents, explosives | Structural modification in the synthesis of various compounds |
| Medical Uses | Antiseptic, anesthetic, precursor in pharmaceutical synthesis | Not used directly, but influences drug design |
| Aromaticity | Aromatic with distinctive reactivity due to hydroxyl group | Aromatic due to presence in benzene ring |
| Role in Reactions | Participates as a reagent in various reactions | Influences reactivity and properties of molecules it’s part of |
| Chemical Properties | Can undergo electrophilic substitution, hydrogen bonding | Nonpolar, lipophilic, minimal direct reactivity |
| Industries | Chemical industry, medicine | Pharmaceutical, agrochemical, materials science |
| Safety | Toxic, requires proper handling and precautions | Generally safe as part of compounds, safety assessment needed |
| Characteristics | Versatile, reactive, acidic | Influential, nonpolar, lipophilic |
| Common Compounds | Phenolic compounds, disinfectants | Drug molecules, agrochemicals, aromatic compounds |
| Examples | Phenol, cresol, resorcinol | Phenylalanine, phenylketonuria (PKU), phenyl group in drug molecules |
In one corner, we have phenol – a compound that seems to hold secrets within its molecular structure. With a hydroxyl group dancing merrily on a benzene ring, phenol’s versatility catches the eye. Its solubility in water, surprising acidity, and reactivity make it a true chameleon in the chemical world. On the other hand, phenyl, though seemingly quieter, boasts its own charm. It’s not a lone warrior but rather a fragment, a part of molecules that wields immense influence. Nonpolar, lipophilic, and a cornerstone of drug design, the phenyl group’s role is as subtle as it is essential.
Differences Between Phenol and Phenyl
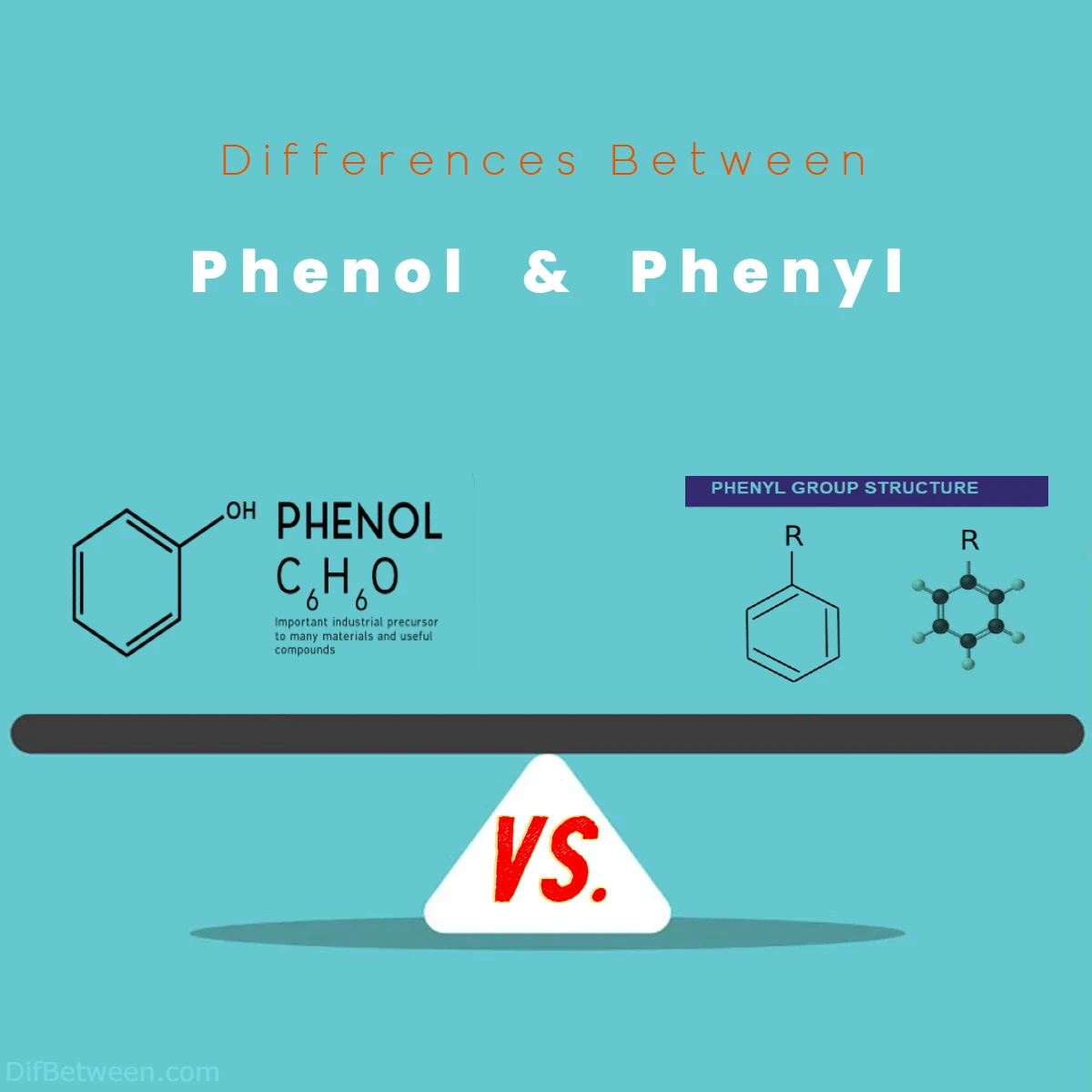
The main differences between Phenol and Phenyl lie in their structural composition and properties. Phenol is a compound featuring a hydroxyl group attached to a benzene ring, imparting unique solubility in water, acidity, and reactivity. In contrast, Phenyl is a fragment derived from a benzene ring by removing a hydrogen atom, making it nonpolar and lipophilic. Phenol exhibits versatility in industrial applications and medical uses, while the Phenyl group plays a crucial role in drug design and agrochemical enhancement. These distinctions highlight the diverse paths these compounds take in the realm of chemistry.
The Basics: Phenol and Phenyl
Phenol: A Glimpse into its Nature Phenol, often referred to as hydroxybenzene, stands as a remarkable compound in the realm of organic chemistry. Its chemical formula, C6H6O, hints at its six carbon and one oxygen atom arrangement. Crucially, phenol boasts a hydroxyl (-OH) group attached to one of its carbon atoms, which imparts distinctive traits to this compound. The hydroxyl group is the crucial factor that sets phenol apart from its cousin, phenyl. This group brings with it a host of reactivity and behavior that distinguishes phenol from other hydrocarbons.
Phenyl: Peering into its Essence Phenyl, on the other hand, represents a functional group consisting of a benzene ring without any substituents or functional groups attached to it. In simpler terms, it’s a part of a molecule where a single hydrogen atom has been removed from a benzene ring. This seemingly subtle alteration can significantly influence the chemical and physical behavior of the compound it’s a part of.
Structural Standpoint: A Deeper Dive
Phenol: Understanding its Structure At the heart of phenol’s distinctiveness lies its structure. Its molecular arrangement includes a six-membered carbon ring, akin to benzene, but with a pivotal alteration—a hydroxyl group (-OH) replaces one of the hydrogen atoms in the benzene ring. This transformation sets the stage for a cascade of differences in properties and behavior compared to compounds like benzene or phenyl.
Phenyl: The Structural Nuance Phenyl, in contrast, isn’t a compound on its own. It’s a fragment, a part of a molecule. Picture a benzene ring with one hydrogen atom extracted; that’s the phenyl group. Its structure is remarkably simple yet holds the power to change the characteristics of the molecule it’s incorporated within. Whether it’s attached to another carbon chain or a complex functional group, the presence of the phenyl group introduces distinct chemical traits.
Properties Speak Volumes: A Comparative Analysis
Phenol: Unveiling its Properties The hydroxyl group in phenol brings about a sea change in its properties. Let’s explore some of its key attributes:
- Solubility: Phenol’s solubility lies in a unique realm. While it’s soluble in water to some extent, unlike hydrocarbons, owing to the hydrogen bonding between the hydroxyl group and water molecules, it doesn’t dissolve as readily as simple alcohols. This distinctive solubility behavior sets phenol apart in the realm of organic compounds.
- Acidity: Prepare to be surprised! Phenol isn’t your run-of-the-mill organic compound; it’s an acid. The hydroxyl group’s presence facilitates the release of a proton, turning phenol into a weak acid. This property isn’t shared by the phenyl group, which lacks the hydroxyl moiety.
- Reactivity: Phenol’s reactivity takes an intriguing turn due to the hydroxyl group. It can partake in hydrogen bonding, halogenation, and nitration reactions. Its unique behavior in these reactions stems from the interplay between the hydroxyl group and the aromatic ring. This makes phenol a key player in the production of numerous industrial chemicals and pharmaceuticals.
Phenyl: Properties in Perspective Now, let’s delve into the properties of the phenyl group and the compounds it influences:
- Nonpolarity: The phenyl group’s nonpolar nature stems from its carbon-carbon and carbon-hydrogen bonds. While the benzene ring itself is prone to exhibiting resonance, the absence of functional groups in the phenyl group results in reduced reactivity compared to compounds with a hydroxyl or carboxyl group.
- Lipophilicity: Due to its nonpolar character, the phenyl group tends to be lipophilic, meaning it has an affinity for lipid-rich environments and nonpolar solvents. This property becomes crucial in the realm of drug design and the interactions of drugs with biological membranes.
Applications: Where They Shine
Phenol: Applications and Utilizations Phenol’s unique properties make it indispensable across various domains:
- Industrial Chemistry: Phenol is a cornerstone of industrial chemistry, finding use in producing plastics, detergents, and even explosives. Its reactivity, especially in processes like nitration and bromination, contributes to the creation of a plethora of essential compounds.
- Medical Sphere: Phenol’s antiseptic and anesthetic properties have earned it a spot in medical applications. It’s used in surgeries and as a disinfectant for skin and surfaces. Additionally, it’s a precursor in the synthesis of pharmaceuticals.
Phenyl: Influential in Pharmaceuticals The phenyl group might lack the spotlight, but its role in pharmaceuticals is indispensable:
- Drug Design: Medicinal chemists harness the phenyl group’s lipophilicity to tailor drug molecules’ interactions with biological systems. By strategically placing phenyl moieties, they enhance a drug’s effectiveness and delivery.
- Agrochemicals: Phenyl-containing compounds contribute to the world of agrochemicals. The introduction of phenyl rings can enhance the bioactivity and selectivity of pesticides and herbicides.
Reactivity and Functionalization
Phenol: A Reactivity Marvel The hydroxyl group in phenol doesn’t just bring acidity; it also grants it a unique reactivity landscape. Phenol’s hydroxyl hydrogen is more acidic than a typical alkyl alcohol, thanks to the aromatic stabilization of the phenoxide ion formed upon deprotonation. This enhanced acidity paves the way for reactions like:
- Electrophilic Substitution: Phenol’s ring is susceptible to electrophilic substitution reactions, where the hydrogen in the hydroxyl group can be replaced by other functional groups. This forms the basis of its role in the synthesis of pharmaceuticals, dyes, and more.
- Kolbe-Schmitt Reaction: Phenol’s reactivity allows it to participate in the Kolbe-Schmitt reaction, leading to the production of salicylic acid. This acid, in turn, serves as a precursor for aspirin, showcasing phenol’s vital role in medicine.
Phenyl: Substitution Dynamics Incorporating a phenyl group into a molecule can dramatically alter its reactivity. Phenyl groups are electron-donating by nature due to their π-electron system, which can influence the behavior of the molecule they’re part of. However, without the hydroxyl group’s reactivity, the phenyl group itself undergoes relatively limited direct chemical transformations.
Safety and Toxicity
Phenol: Handle with Care While phenol’s versatility is impressive, it’s essential to note its toxicity. Phenol’s strong caustic properties can lead to severe burns upon contact with skin, making proper handling crucial in industrial and laboratory settings. Inhalation of phenol vapors can also cause respiratory distress. However, its antiseptic qualities have led to its use in lower concentrations in products like throat sprays and mouthwashes.
Phenyl: Milder Persona The phenyl group, on its own, doesn’t exhibit the same level of toxicity as phenol. However, its presence in various compounds should still be considered when assessing their safety. In pharmaceutical contexts, the inclusion of phenyl moieties might influence drug metabolism, and this aspect requires careful consideration during drug development.
Synthetic Significance
Phenol: Catalyst and Precursor Phenol’s reactivity extends to catalytic applications. It can serve as a catalyst in various reactions, including the synthesis of cyclohexanone from cyclohexanol via the cumene process. Its role as a precursor for resins and adhesives also underscores its significance in industrial chemistry.
Phenyl: Scaffold for Diversity Phenyl rings act as versatile scaffolds in synthetic chemistry. By attaching different functional groups to the phenyl group, chemists can create a myriad of compounds with varying properties. This diversity finds applications in materials science, drug discovery, and agrochemicals.
A Glimpse into Aromaticity
Phenol: Aromatic Yet Distinct Both phenol and phenyl are rooted in the world of aromatic compounds, which possess a stabilized ring system due to electron delocalization. Phenol’s unique blend of aromaticity and the hydroxyl group’s influence on its reactivity presents a delicate balance between traditional aromatic compounds and the more reactive phenolic derivatives.
Phenyl: Aromatic Essence The phenyl group, being a part of the benzene ring, embodies aromaticity at its core. This electron-rich ring system contributes to the stability of molecules containing phenyl groups and their resonance structures. This aspect, combined with its lipophilic nature, significantly impacts the interactions of these compounds in biological systems.
Phenol or Phenyl: Which One is Right for You?
As you navigate the intricate world of chemistry, you’re likely to encounter compounds that appear quite similar at first glance but possess unique traits that set them apart. Phenol and phenyl are two such terms that might pique your curiosity. But fear not, for I’m here to guide you through the decision-making process by unveiling the distinctive features of phenol and phenyl. Let’s embark on this enlightening journey to help you determine which one might be the right choice for your specific needs.
Understanding the Basics: Phenol and Phenyl
Phenol: A World of Versatility Phenol, with its hydroxyl group snuggled into a benzene ring, is a compound that boasts a remarkable level of versatility. This hydroxyl group brings with it unique traits, including solubility to some extent in water and a surprising acidity that makes phenol a weak acid. This compound’s reactivity is a game-changer, allowing it to participate in various reactions such as electrophilic substitution and the intriguing Kolbe-Schmitt reaction. If you’re looking for a compound that dances between solubility and reactivity, phenol might be your go-to choice.
Phenyl: A Silent Influencer On the other hand, phenyl isn’t a compound on its own but a part of one. Imagine a benzene ring with a single hydrogen atom missing, and that’s the phenyl group. It might seem unassuming, but this fragment wields significant influence. It’s nonpolar and lipophilic, making it a great fit for drug design and agrochemical applications. If you’re seeking a structural element that quietly enhances the properties of a molecule, the phenyl group could be your ideal companion.
When to Choose Phenol: Unveiling the Scenarios
Phenol’s Spotlight: Industrial and Medical Marvel
- Industrial Marvel: If your interest lies in the realm of industrial chemistry, phenol might be the star you’re seeking. Its reactivity opens doors to diverse applications in producing plastics, detergents, and explosives. Its participation in processes like nitration and bromination contributes to the creation of essential compounds in the chemical industry.
- Medicine’s Ally: Phenol’s antiseptic and anesthetic properties have earned it a place in medical applications. It’s used in surgeries, disinfectants, and even as a precursor in pharmaceutical synthesis. If the medical field beckons, phenol stands ready to play its part.
When to Choose Phenyl: Crafting Molecules with Precision
Phenyl’s Potential: Design and Agrochemicals
- Tailoring Drug Design: Are you delving into the intricate world of drug design? The phenyl group might be your guiding light. Its lipophilic nature allows it to modulate how drugs interact with biological systems. By strategically placing phenyl moieties, you can enhance drug effectiveness and delivery, paving the way for groundbreaking pharmaceuticals.
- Agrochemical Enhancement: When agrochemicals are your focus, the phenyl group shines bright. Incorporating phenyl rings into pesticide and herbicide molecules can enhance bioactivity and selectivity. If agriculture’s challenges call out to you, the phenyl group has answers.
The Decision: Phenol or Phenyl?
As you stand at this crossroads of phenol and phenyl, your choice depends on your aspirations and goals. Are you captivated by the diverse applications of a reactivity-rich compound that dances on the edge of acidity? Phenol might be your companion. Or does your journey involve sculpting molecules with precision, be it in drug design or agrochemical innovation? In that case, the phenyl group’s subtle influence might be your ideal ally.
FAQs
The primary distinction lies in their composition and functionality. Phenol is a compound consisting of a hydroxyl group attached to a benzene ring, contributing to its unique solubility, acidity, and reactivity. On the other hand, Phenyl is a fragment derived from a benzene ring by removing a hydrogen atom, rendering it nonpolar and lipophilic.
Phenol exhibits solubility in water due to hydrogen bonding, while Phenyl is insoluble and nonpolar. Phenol’s hydroxyl group grants it weak acidity and reactivity, allowing it to partake in various chemical reactions. In contrast, the Phenyl group’s influence lies in its lipophilicity and capacity to modulate interactions in drug design and agrochemical contexts.
Phenol finds applications in industrial chemistry, contributing to the production of plastics, detergents, and disinfectants. It’s also a precursor in pharmaceutical synthesis. Phenyl’s role is significant in drug design, enhancing lipophilicity for effective interactions in drug molecules, and in agrochemical development to improve bioactivity and selectivity.
Phenol can be caustic and toxic, especially in concentrated forms, necessitating careful handling. Phenyl, when part of compounds, is generally safe, but its presence should be assessed for safety in various contexts.
Phenol remains aromatic due to its benzene ring and the hydroxyl group’s influence. Phenyl, being a part of the benzene ring, also contributes to aromaticity and stability within molecules.
No, they serve distinct roles. Phenol’s reactivity and solubility make it suitable for various applications, while the Phenyl group’s lipophilicity is valuable for modifying properties in drug molecules and agrochemicals.
The choice depends on your specific goals. If you need reactivity and solubility, phenol might be ideal. If you’re aiming for lipophilicity and structural enhancement, the Phenyl group could be more fitting.
Both compounds enrich the field with their unique properties. Phenol’s versatility impacts industrial and medical sectors, while the Phenyl group’s subtler influence is pivotal in drug design and agrochemical development.
Phenol examples include various phenolic compounds used in disinfectants and pharmaceutical synthesis. Phenyl is found in compounds like phenylalanine (an amino acid) and within drug molecules where it enhances interactions.
Phenol’s distinctiveness arises from its hydroxyl group, leading to solubility, acidity, and reactivity, while Phenyl’s influence lies in its nonpolarity, lipophilicity, and subtle yet powerful role in modifying properties in various applications.
Read More: