| Characteristic | Iodine | Potassium Iodide |
|---|---|---|
| Chemical Formula | I2 | KI |
| Molecular Weight (g/mol) | 253.8 | 166 |
| Physical Form | Purple-black crystals | White crystalline salt |
| Melting Point (°C) | 113.7 | 681 |
| Boiling Point (°C) | 184.3 | 1330 |
| Solubility in Water | Slightly soluble | Highly soluble |
| Natural Occurrence | In seaweed and seafood | Primarily synthetic |
| Main Sources | Seaweed, seafood | Iodized salt, pharmaceuticals |
| Primary Applications | Antiseptics, dietary supplements,chemical reactions | Radiation protection, pharmaceuticals,iodized salt |
| Health Benefits | Thyroid function, prevention of iodine deficiency disorders | Thyroid protection, emergency preparedness |
| Health Risks | Excessive intake can lead to thyroid dysfunction | Possible side effects (e.g., gastrointestinal symptoms, allergies) |
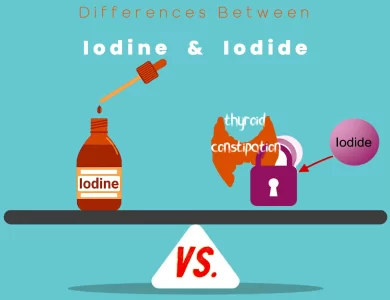
In the realm of science and wellness, understanding the differences between iodine and potassium iodide can be empowering. It allows you to make informed decisions about your health and safety, whether you’re considering the benefits of iodine supplementation or pondering the importance of potassium iodide in radiation protection.
Differences Between Iodine and Potassium Iodide
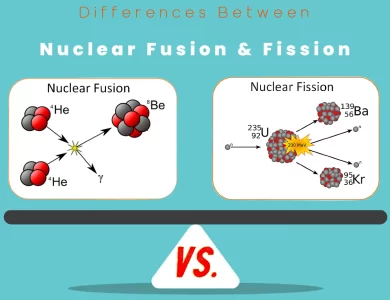
The main differences between Iodine and Potassium Iodide lie in their chemical composition, sources, and applications. Iodine exists as a purple-black diatomic molecule with a relatively low boiling point, primarily found in seaweed and seafood. It is utilized in medical antiseptics, dietary supplements, and chemical reactions. In contrast, Potassium Iodide is a white crystalline salt, synthetically produced, often used for radiation protection in emergencies, as a component in pharmaceuticals, and in the fortification of table salt to prevent iodine deficiency. Understanding these distinctions is essential for choosing the right substance for specific needs, be it for health or industrial purposes.
Chemical Composition
Iodine:
Iodine, symbolized as I on the periodic table, is a chemical element with atomic number 53. In its pure form, iodine exists as a diatomic molecule (I2) with a distinctive purple-black color. It has a molecular weight of approximately 253.8 g/mol.
Iodine is a non-metal and belongs to the halogen group, which also includes fluorine, chlorine, bromine, and astatine. This element has a relatively low melting point (-114 degrees Celsius) and a boiling point of 184 degrees Celsius.
Potassium Iodide:
Potassium iodide, often abbreviated as KI, is a chemical compound made up of potassium (K) and iodine (I). Its chemical formula is KI, and it has a molecular weight of approximately 166 g/mol.
Potassium iodide is a white, crystalline salt that is highly soluble in water. It is typically found as a solid or in solution form. This compound is widely used in various applications, especially in the medical and pharmaceutical industries.
Sources and Occurrence
Iodine:
Iodine is a relatively rare element in Earth’s crust, with an average abundance of about 0.46 grams per ton. It is not found in its pure elemental form in nature due to its high reactivity. Instead, iodine is primarily present as iodide ions (I-) in various minerals and as a trace element in seawater.
The main natural source of iodine is the ocean, where it is found in dissolved form as iodide ions. Seaweed and seafood, such as fish and shellfish, are rich dietary sources of iodine because they accumulate iodine from seawater.
Potassium Iodide:
Potassium iodide, on the other hand, is a synthetic compound produced by combining potassium hydroxide (KOH) with iodine (I2). It is not commonly found in nature in significant quantities and is primarily manufactured for specific applications.
While potassium iodide itself is not a natural source of iodine, it is a crucial component in the fortification of table salt with iodine, which is done to prevent iodine deficiency disorders in populations that lack access to iodine-rich foods.
Uses and Applications
Iodine:
Iodine finds a wide range of applications across various industries and fields:
- Medical Applications: Iodine is used in the form of iodine-based antiseptics like povidone-iodine for disinfection of skin and mucous membranes before surgical procedures.
- Nutritional Supplement: Iodine is an essential micronutrient required for the synthesis of thyroid hormones. It is added to some dietary supplements and iodized salt to prevent iodine deficiency.
- Chemical Industry: Iodine is used in various chemical reactions, including the preparation of certain organic compounds and reagents.
- Photography: Silver iodide (AgI) is used in photographic emulsions as a light-sensitive material.
Potassium Iodide:
Potassium iodide has specific applications distinct from iodine:
- Radiation Emergency: Potassium iodide is well-known for its role in radiation emergencies. It is used to protect the thyroid gland from absorbing radioactive iodine, which can be released during nuclear accidents or radiation therapy.
- Pharmaceuticals: Potassium iodide is used in some medications, particularly those for treating thyroid disorders like hyperthyroidism.
- Iodized Salt: Potassium iodide is added to table salt as a means of iodine fortification. This has been a successful public health initiative to prevent iodine deficiency disorders.
- Chemical Reagent: In laboratories, potassium iodide is used as a reagent in various chemical tests and experiments.
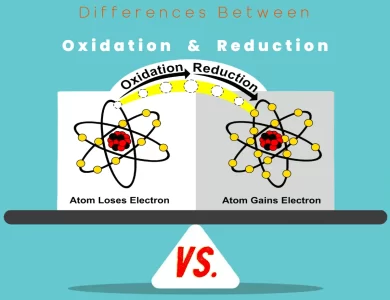
Health Benefits and Risks
Iodine:
Iodine is essential for the proper functioning of the human body, primarily due to its role in thyroid hormone production. Here are the health benefits and risks associated with iodine:
Health Benefits:
- Thyroid Health: Iodine is a crucial component of thyroid hormones (thyroxine and triiodothyronine), which regulate metabolism and overall growth and development.
- Iodine Deficiency Prevention: Adequate iodine intake helps prevent iodine deficiency disorders, such as goiter and cretinism.
Risks:
- Excessive Intake: Consuming too much iodine can lead to thyroid dysfunction and other health issues. It’s essential to maintain a balanced intake.
Potassium Iodide:
Potassium iodide primarily offers benefits in specific situations, particularly related to radiation exposure. Here are the health benefits and risks associated with potassium iodide:
Health Benefits:
- Radiation Protection: Potassium iodide can protect the thyroid gland from absorbing radioactive iodine isotopes, reducing the risk of radiation-induced thyroid cancer.
- Emergency Preparedness: It is a critical component of emergency preparedness kits for individuals living near nuclear facilities or in areas prone to nuclear accidents.
Risks:
- Side Effects: Like any medication, potassium iodide may have side effects, including gastrointestinal symptoms and allergic reactions.
Chemical Properties
Iodine:
Iodine, in its pure elemental form (I2), is known for its unique purple-black crystals. This characteristic color is due to the interaction of light with the electron structure of the iodine molecule. Interestingly, iodine can sublimate, meaning it can change directly from a solid to a gas without passing through a liquid phase. This property is commonly demonstrated by the production of violet-colored vapor when solid iodine is heated.
In addition to its distinctive color, iodine is also known for its strong odor and volatile nature. It readily vaporizes at room temperature, filling the air with its pungent smell.
Potassium Iodide:
Potassium iodide, in contrast, appears as white crystalline salt. It has a much milder odor compared to pure iodine and is highly soluble in water. This solubility makes it suitable for use in liquid formulations, such as iodine solutions and medications.
The white, solid nature of potassium iodide makes it easy to handle and incorporate into various applications. Its solubility allows for precise dosing in medical and pharmaceutical settings.
Health Implications
Iodine:
Iodine is essential for thyroid health, as it is a key component of thyroid hormones. Thyroid hormones play a critical role in regulating metabolism, energy production, and overall growth and development. Without adequate iodine, the thyroid gland cannot produce these hormones, leading to various health issues.
On the flip side, excessive iodine intake can be detrimental to thyroid function. The thyroid can become overactive or underactive, causing conditions like hyperthyroidism or hypothyroidism. Striking the right balance in iodine intake is crucial for maintaining optimal thyroid function.
Potassium Iodide:
Potassium iodide’s primary health implication is related to its role in radiation protection. In the event of a nuclear accident or exposure to radioactive iodine isotopes, the thyroid gland can absorb these harmful isotopes. This absorption can lead to an increased risk of thyroid cancer.
Potassium iodide works by saturating the thyroid gland with non-radioactive iodine, preventing the uptake of radioactive iodine. This is a crucial measure in radiation emergency preparedness, as it can significantly reduce the risk of thyroid damage and cancer in exposed individuals.
Lesser-Known Applications
Iodine:
Beyond its well-known uses in healthcare and nutrition, iodine has some lesser-known applications:
- Water Treatment: Iodine is used as a water disinfectant and purification agent, particularly in situations where access to clean water is limited.
- Analytical Chemistry: Iodine is employed in analytical chemistry as a titration reagent and for the detection of certain analytes.
- Stain for Biological Specimens: Iodine solutions, such as Lugol’s iodine, are used in laboratories to stain biological specimens for microscopy.
- Iodine Clock Reaction: Iodine is featured in chemical demonstrations, like the famous “iodine clock reaction,” to showcase chemical kinetics.
Potassium Iodide:
While potassium iodide’s primary role is in radiation protection and as a pharmaceutical ingredient, it also finds application in some niche areas:
- Pyrotechnics: Potassium iodide is used in fireworks and pyrotechnics to produce violet-colored flames.
- Analytical Chemistry: In laboratories, potassium iodide is used as a source of iodine ions in redox titrations and as a reducing agent.
- Art Conservation: Potassium iodide can be employed in art conservation to treat and stabilize certain types of paper and artworks.
- Photography: In addition to silver iodide, potassium iodide is used in the photographic industry to sensitize emulsions.
Safety Considerations
Iodine:
Handling pure iodine requires caution due to its volatile nature and staining properties. Direct contact with the skin or mucous membranes can lead to irritation and staining. Moreover, inhaling iodine vapor should be avoided, as it can irritate the respiratory system.
When used for medical purposes or as a dietary supplement, iodine is typically in a controlled and safe form, such as iodine tinctures or iodized salt. These formulations ensure precise dosing and minimize potential risks.
Potassium Iodide:
Potassium iodide is generally considered safe when used as directed, especially in the context of radiation emergency preparedness or medical treatment. However, like any medication, it may have potential side effects, including gastrointestinal symptoms (e.g., upset stomach) and allergic reactions in some individuals.
Iodine or Potassium Iodide: Which One is Right Choose for You?
Choosing between iodine and potassium iodide depends on your specific needs and intended applications. Let’s break down the decision-making process to help you determine which one is right for you:
When to Choose Iodine:
- General Disinfection: If you need a disinfectant for skin, wounds, or mucous membranes, iodine-based antiseptics, such as povidone-iodine, are effective choices. They are widely used in healthcare settings for pre-surgical skin preparation and wound care.
- Nutritional Supplementation: When you require iodine as a dietary supplement to maintain optimal thyroid function and prevent iodine deficiency disorders, consider iodine supplements. These supplements come in various forms, including iodine tinctures and iodized salt.
- Chemical Reactions: If you’re conducting chemical experiments or reactions that specifically require iodine, you’ll need pure iodine. It can be used as a reagent in various laboratory procedures.
- Photography: In the world of photography, silver iodide (AgI) is used as a light-sensitive material in photographic emulsions. If you’re an enthusiast or professional photographer, iodine’s role is in the production of photographic materials.
When to Choose Potassium Iodide:
- Radiation Protection: In the event of a nuclear accident or exposure to radioactive iodine isotopes, potassium iodide is crucial. It protects the thyroid gland from absorbing harmful radioactive iodine. If you live near a nuclear facility or in an area prone to nuclear accidents, having potassium iodide on hand is advisable.
- Thyroid Medication: If you’re prescribed medication for thyroid disorders like hyperthyroidism, some medications may contain potassium iodide as an active ingredient. Follow your healthcare provider’s recommendations for its use.
- Iodized Salt: Potassium iodide is a key component in iodized salt, a public health initiative aimed at preventing iodine deficiency disorders. If you want to ensure you’re getting enough iodine in your diet, using iodized salt is a simple way to do so.
- Emergency Preparedness: Potassium iodide should be part of your emergency preparedness kit if you live in an area with potential radiation hazards. Being ready for unforeseen events is essential, and potassium iodide can provide a vital layer of protection.
Final Considerations:
In most cases, the choice between iodine and potassium iodide is clear-cut based on your specific requirements. However, it’s crucial to exercise caution and use these substances as directed. Both iodine and potassium iodide have potential risks if mishandled or used improperly.
Always follow recommended dosages and guidelines, especially when using them for medical or emergency purposes. If you’re unsure about which option is best for your situation, consult with a healthcare professional or a chemist who can provide expert guidance based on your needs.
FAQs
The primary difference lies in their chemical composition and applications. Iodine exists as a purple-black diatomic molecule and is used in antiseptics, dietary supplements, and chemical reactions. Potassium iodide, on the other hand, is a white crystalline salt, often utilized for radiation protection, in pharmaceuticals, and in iodized salt to prevent iodine deficiency.
Yes, both have health benefits. Iodine is crucial for thyroid function and preventing iodine deficiency disorders. Potassium iodide is vital for thyroid protection in radiation emergencies and as a dietary iodine source through iodized salt.
Not necessarily. Their different properties and applications make them suited for distinct purposes. Iodine is commonly used in skin disinfection and dietary supplements, while potassium iodide is primarily employed in radiation protection and iodized salt.
Excessive iodine intake can lead to thyroid dysfunction. Potassium iodide may have side effects like gastrointestinal symptoms and allergies in some individuals. Proper dosing and usage are essential to minimize risks.
Iodine can be found naturally in seaweed and seafood. Potassium iodide, however, is primarily synthesized and is not commonly found in nature in significant quantities.
Read More:
Contents