| Characteristic | Oxidation | Reduction |
|---|---|---|
| Definition | Loss of electrons | Gain of electrons |
| Electron Transfer | Electrons are lost by the substance being oxidized | Electrons are gained by the substance being reduced |
| Oxidation State Change | Increases (becomes more positive) | Decreases (becomes more negative) |
| Involvement of Oxygen | May involve the addition of oxygen atoms | May involve the removal of oxygen atoms |
| Involvement of Hydrogen | May involve the removal of hydrogen atoms | May involve the addition of hydrogen atoms |
| Example in Biological Systems | Occurs during processes like cellular respiration | Occurs during processes like photosynthesis |
| Common Examples | Rusting of iron, combustion of fuels, food spoilage | Bioremediation, metal production, use of antioxidants |
| Electron Distribution | Electrons are distributed away from the oxidized species | Electrons are distributed away from the reduced species |
| Role in Chemistry | Often associated with the degradation or breakdown of compounds | Often associated with the synthesis or formation of compounds |
| Impact on Reactivity | Tends to increase the reactivity of a substance | Tends to decrease the reactivity of a substance |
| Symbolic Representation | Typically represented with a positive sign (+) for the change in oxidation state | Typically represented with a negative sign (-) for the change in oxidation state |
| Electrochemistry | Occurs at the anode in electrochemical cells | Occurs at the cathode in electrochemical cells |
| Biological Significance | Essential for breaking down nutrients to produce energy | Crucial for building complex molecules and storing energy |
| Symbolic Representation | Typically represented with a positive sign (+) for the change in oxidation state | Typically represented with a negative sign (-) for the change in oxidation state |
As we navigate the intricate pathways of chemical transformations, we’ll uncover how oxidation and reduction are interwoven in the fabric of our existence. From rusting iron to the energy production in our cells, these processes leave their mark on everything we see and touch.
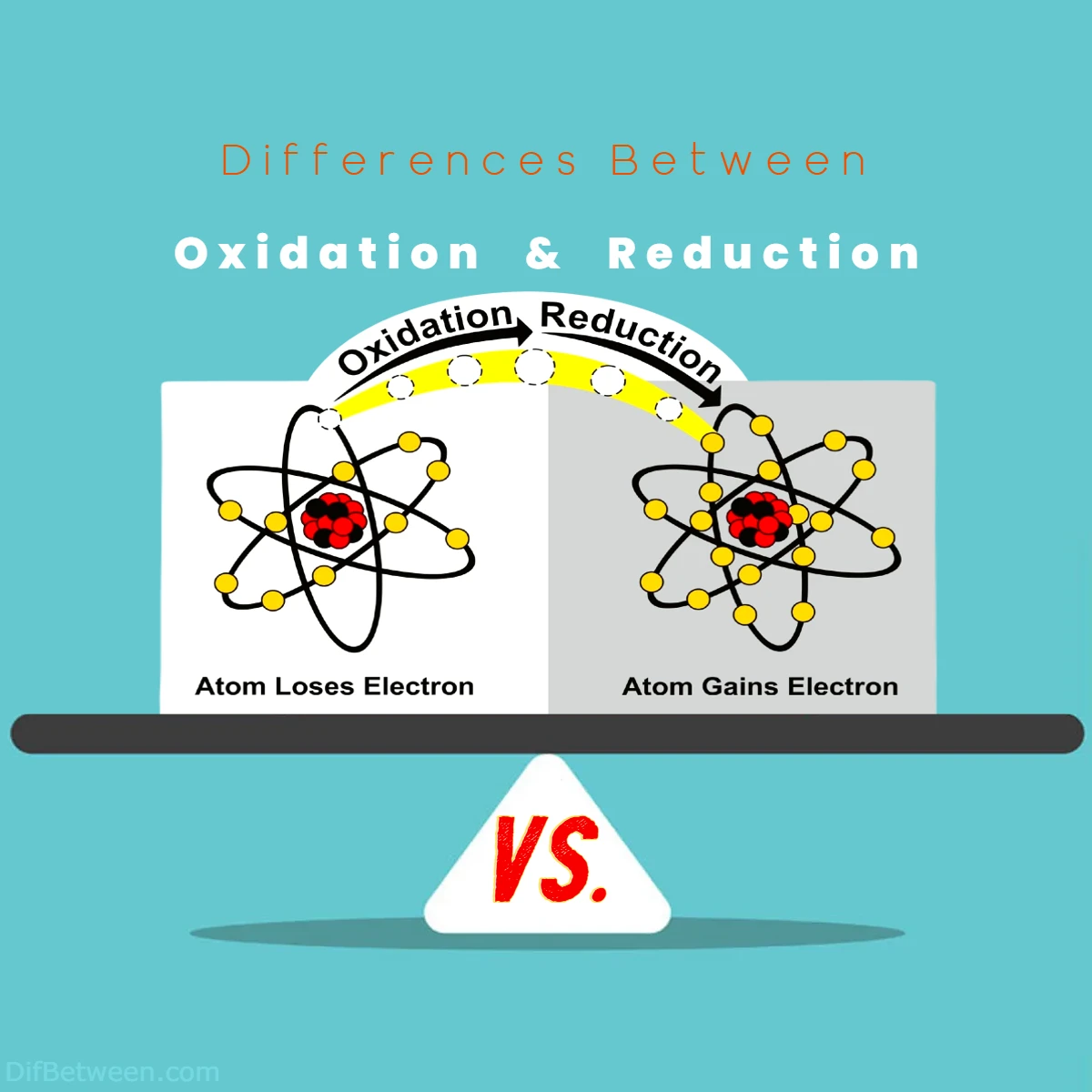
Differences Between Oxidation and Reduction
The main differences between Oxidation and Reduction lie in their electron behavior. Oxidation involves the loss of electrons, resulting in an increase in oxidation state, while Reduction is characterized by the gain of electrons, leading to a decrease in oxidation state. These processes are interconnected in redox reactions, where one substance gets oxidized as it loses electrons, and another gets reduced as it gains electrons. Oxidation often involves the addition of oxygen or the removal of hydrogen, while Reduction can entail the addition of hydrogen or the removal of oxygen. Understanding these distinctions is crucial in comprehending chemical reactions, from rust formation to energy production in living organisms.
Defining
Oxidation: Embracing the Loss
Oxidation, often referred to as the “loss of electrons,” is a chemical process where an atom, ion, or molecule loses one or more electrons. It’s like an unfortunate game of pass-the-parcel where one electron gets passed from one participant to another. This loss of electrons typically occurs in conjunction with an increase in the atom’s oxidation state or oxidation number.
Example of Oxidation:
Imagine a sodium (Na) atom. When it reacts with chlorine (Cl) to form sodium chloride (NaCl), sodium loses an electron to chlorine. This loss of an electron transforms sodium from a neutral atom (Na) into a positively charged ion (Na+). This transition from Na to Na+ represents oxidation.
Reduction: Embracing the Gain
On the flip side, we have reduction, often described as the “gain of electrons.” In this process, an atom, ion, or molecule acquires one or more electrons, reducing its oxidation state or oxidation number. Reduction is like winning the electron lottery, with atoms getting richer in negatively charged electrons.
Example of Reduction:
Continuing with our sodium chloride (NaCl) example, when chlorine (Cl) gains an electron from sodium (Na), it transforms from a neutral atom (Cl) into a negatively charged ion (Cl-). This transition from Cl to Cl- represents reduction.
Oxidation and Reduction: Two Sides of the Same Coin
Now that we’ve defined oxidation and reduction, let’s explore their fundamental characteristics and how they are interconnected.
Redox Reactions: The Marriage of Oxidation and Reduction
In the world of chemistry, oxidation and reduction often come hand in hand, forming what we call redox (reduction-oxidation) reactions. These reactions involve the transfer of electrons from one substance to another, creating a fascinating interplay of gain and loss.
In a redox reaction, one substance is oxidized, losing electrons and increasing its oxidation state, while another substance is reduced, gaining electrons and decreasing its oxidation state. It’s like a cosmic dance where electrons change partners, and the chemical world transforms.
Example of a Redox Reaction:
Consider the combustion of methane (CH4) in the presence of oxygen (O2) to produce carbon dioxide (CO2) and water (H2O). In this reaction, methane is oxidized, and oxygen is reduced.
Oxidation: CH4 → CO2 + 2H2O
In this part of the reaction, methane loses hydrogen atoms, and its carbon atom becomes more oxidized, going from an oxidation state of -4 to +4.
Reduction: O2 → 2H2O
In this part of the reaction, oxygen gains electrons from hydrogen, changing from its diatomic state (O2) to water (H2O).
Oxidation Numbers: Tracking the Charge
To understand oxidation and reduction better, chemists use a concept called oxidation numbers. Oxidation numbers are assigned to atoms in a compound to keep track of their electron distribution and determine if they are oxidized or reduced during a reaction.
Here are some key rules for assigning oxidation numbers:
- Elemental Forms: In their elemental forms, atoms have an oxidation number of zero. For example, O2, H2, and N2 all have oxidation numbers of zero.
- Monatomic Ions: The oxidation number of a monatomic ion is equal to its charge. For example, the oxidation number of Na+ is +1, and the oxidation number of Cl- is -1.
- Hydrogen (H): In most compounds, hydrogen is assigned an oxidation number of +1. However, in metal hydrides like NaH, hydrogen has an oxidation number of -1.
- Oxygen (O): In most compounds, oxygen is assigned an oxidation number of -2. There are exceptions, such as peroxides (like H2O2), where oxygen has an oxidation number of -1.
- Sum of Oxidation Numbers: In a neutral compound, the sum of the oxidation numbers of all the atoms must equal zero. In a polyatomic ion, the sum of the oxidation numbers must equal the ion’s charge.
Let’s apply these rules to some examples to determine oxidation numbers:
Example 1: In H2O (water), hydrogen is assigned +1, and oxygen is assigned -2. The sum of the oxidation numbers is 2(+1) + (-2) = 0, satisfying the rule for a neutral compound.
Example 2: In H2O2 (hydrogen peroxide), hydrogen is still +1, but oxygen is assigned -1. The sum of the oxidation numbers is 2(+1) + 2(-1) = 0, again satisfying the rule for a neutral compound.
Example 3: In SO4^2- (sulfate ion), oxygen is still -2, and the sum of the oxidation numbers must equal -2, which is the charge of the ion. Therefore, sulfur must have an oxidation number of +6 to balance the equation: 4(-2) + x = -2.
The Oxidation State vs. The Formal Charge
While oxidation numbers are a helpful tool for tracking electron distribution in compounds, it’s important to distinguish them from formal charges. Oxidation numbers are assigned based on a set of rules and are a simplified way to understand electron distribution in molecules. In contrast, formal charges are a more detailed and specific method used to assess the electron distribution within individual atoms in a molecule.
Oxidation State: The oxidation state of an atom is assigned based on rules and guidelines, and it represents the apparent charge on that atom in a compound. Oxidation states are used to determine if an atom is oxidized or reduced during a chemical reaction.
Formal Charge: Formal charges are calculated by comparing the number of valence electrons an atom “should” have (based on its position in the periodic table) with the number of electrons it actually has in a molecule. Formal charges are used to evaluate the distribution of electrons within a molecule and assess the stability of its Lewis structure.
Here’s a quick comparison of oxidation states and formal charges:
| Oxidation State | Formal Charge | |
|---|---|---|
| Calculation Basis | Rules and guidelines for assigning oxidation numbers. | Comparison between valence electrons and actual electrons in a molecule. |
| Purpose | To track electron distribution in compounds and identify oxidation or reduction. | To evaluate electron distribution within individual atoms in a molecule and assess Lewis structures. |
| Example | In H2O, oxygen has an oxidation state of -2. | In H2O, oxygen has a formal charge of -1. |
In summary, while both oxidation states and formal charges provide insights into electron distribution, oxidation states are broader and are used to understand the overall redox behavior of a compound, while formal charges are more atom-specific and help in fine-tuning molecular structures.
Oxidation and Reduction in Everyday Life
Now that we’ve covered the fundamentals of oxidation and reduction, let’s delve into some real-world examples and applications of these processes that are all around us.
Oxidation in Action: Rusting and Corrosion
One of the most common and visible examples of oxidation in our daily lives is rusting. When iron (Fe) and oxygen (O2) come into contact with water (H2O), a slow but relentless oxidation process occurs, leading to the formation of iron oxide, commonly known as rust (Fe2O3).
The chemical reaction for the rusting of iron can be summarized as follows:
4Fe + 3O2 + 6H2O → 4Fe(OH)3
In this reaction, iron (Fe) is oxidized from its metallic form to iron ions (Fe3+), while oxygen is reduced from its diatomic molecular form (O2) to hydroxide ions (OH-). The formation of rust not only weakens the iron structure but also leads to the iconic reddish-brown appearance associated with rusted metal.
Rust is a pervasive issue with far-reaching consequences. It can compromise the structural integrity of buildings, bridges, and vehicles, costing billions in maintenance and repairs each year. That’s the power of oxidation in action.
Reduction in Metabolism: Cellular Energy Production
While oxidation is often associated with rust and deterioration, reduction plays a vital role in our bodies, particularly in the field of biochemistry. Reduction reactions are integral to cellular energy production through processes like cellular respiration.
In cellular respiration, glucose (C6H12O6) undergoes a series of complex chemical reactions involving both oxidation and reduction to produce energy in the form of adenosine triphosphate (ATP). Here’s a simplified representation of the overall process:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy (as ATP)
In this process, glucose is oxidized, losing electrons, and oxygen is reduced, gaining electrons. The transfer of electrons through various intermediate molecules, such as NADH and FADH2, drives the synthesis of ATP, the cellular energy currency. Without reduction reactions, our cells would struggle to produce the energy required for basic functions and activities.
Oxidation in Food Preservation: Antioxidants
In the world of food preservation, oxidation can be both a friend and a foe. When food undergoes oxidation, it can lead to spoilage, rancidity, and the degradation of nutrients. This is a common concern for those who want to keep their food fresh for longer periods.
To combat the detrimental effects of oxidation, food scientists turn to antioxidants. Antioxidants are compounds that inhibit or slow down the oxidation of food components, such as fats and vitamins. They work by donating electrons to free radicals, highly reactive molecules that can initiate oxidation reactions.
Some common antioxidants used in food preservation include vitamin C (ascorbic acid) and vitamin E (tocopherol). These antioxidants neutralize free radicals and help extend the shelf life of various food products, from oils and snacks to fresh fruits and vegetables.
Reduction in Environmental Cleanup: Bioremediation
In the realm of environmental science and remediation, reduction processes are harnessed for the cleanup of contaminated sites. This approach, known as bioremediation, relies on microorganisms that are capable of reducing harmful pollutants into less toxic or non-toxic forms.
One notable example is the bioremediation of chlorinated solvents like trichloroethylene (TCE). TCE is a common groundwater contaminant found at industrial sites. Certain bacteria, such as Dehalococcoides, are known to perform reductive dechlorination, a process that involves the reduction of TCE to less harmful compounds, ultimately leading to the production of ethene (C2H4) and chloride ions (Cl-).
The overall reaction for the bioremediation of TCE can be summarized as follows:
3C2HCl3 + 2H2O → 2C2H4 + 6HCl
In this reaction, the reduction of TCE to ethene is the key step, rendering the pollutant less harmful to the environment. Bioremediation is a sustainable and environmentally friendly approach to addressing contamination issues, harnessing the power of reduction processes in nature.
Oxidation vs. Reduction: A Comparative Overview
To gain a deeper understanding of the differences between oxidation and reduction, let’s explore various aspects in a side-by-side comparison.
1. Electron Transfer
Oxidation: In oxidation, there is a loss of electrons by an atom, ion, or molecule. This process results in an increase in the oxidation state or oxidation number.
Reduction: In reduction, there is a gain of electrons by an atom, ion, or molecule, leading to a decrease in the oxidation state or oxidation number.
2. Involvement of Oxygen
Oxidation: Oxidation reactions often involve the addition of oxygen to a substance or the removal of hydrogen from it.
Reduction: Reduction reactions typically involve the addition of hydrogen to a substance or the removal of oxygen from it.
3. Example in Biological Systems
Oxidation: Oxidation reactions are crucial in cellular respiration, where glucose is oxidized to produce energy.
Reduction: Reduction reactions play a role in photosynthesis, where carbon dioxide is reduced to form glucose.
4. Common Examples
Oxidation: Rusting of iron, combustion of fuels, and spoilage of food due to exposure to air are common examples of oxidation.
Reduction: Bioremediation of contaminants, the production of metals from their ores, and the use of antioxidants in food preservation are common examples of reduction.
5. Electron Distribution
Oxidation: In oxidation, electrons are distributed away from the oxidized species (which loses electrons) and toward the reduced species (which gains electrons).
Reduction: In reduction, electrons are distributed away from the reduced species (which gains electrons) and toward the oxidized species (which loses electrons).
6. Role in Chemistry
Oxidation: Oxidation reactions often result in the degradation or breakdown of compounds.
Reduction: Reduction reactions are frequently associated with the synthesis or formation of compounds.
7. Impact on Reactivity
Oxidation: Oxidation tends to increase the reactivity of a substance as it loses electrons.
Reduction: Reduction tends to decrease the reactivity of a substance as it gains electrons.
8. Symbolic Representation
Oxidation: Oxidation is often represented with a positive sign (+) for the change in oxidation state.
Reduction: Reduction is often represented with a negative sign (-) for the change in oxidation state.
9. Electrochemistry
Oxidation: In electrochemical cells, the anode is where oxidation occurs.
Reduction: In electrochemical cells, the cathode is where reduction occurs.
10. Biological Significance
Oxidation: Oxidation reactions are essential for breaking down nutrients to produce energy in living organisms.
Reduction: Reduction reactions are crucial for building complex molecules and storing energy in living organisms.
Redox Reactions Unveiled
Redox reactions, short for reduction-oxidation reactions, are at the heart of countless chemical transformations. These reactions involve the transfer of electrons from one substance to another. As electrons are shuttled from one participant to another, the reactants change their oxidation states, and the world of chemistry comes alive.
Oxidation: The Electron Donor
In a redox reaction, there’s always an element or compound that undergoes oxidation, giving up electrons. This process transforms the element or compound into a more positively charged species, often an ion or a molecule with a higher oxidation state.
Let’s consider a classic example: the oxidation of hydrogen peroxide (H2O2) to water (H2O) and oxygen (O2). Here’s the simplified equation:
2H2O2 → 2H2O + O2
In this reaction, hydrogen peroxide loses oxygen atoms and undergoes oxidation. It transitions from a compound with an oxidation state of -1 to one with an oxidation state of -2 (water) and zero (oxygen gas). The hydrogen peroxide serves as the electron donor, providing the electrons for the reduction half of the reaction.
Reduction: The Electron Acceptor
On the flip side, there’s an element or compound that undergoes reduction, gaining electrons. This process transforms the element or compound into a more negatively charged species, often an ion or a molecule with a lower oxidation state.
Continuing with our hydrogen peroxide example, oxygen gas (O2) is the species that undergoes reduction. It gains electrons from the hydrogen peroxide and goes from its diatomic molecular form (O2) to negatively charged hydroxide ions (OH-).
The Balance of Electrons
Redox reactions are all about achieving a delicate balance between oxidation and reduction. For every electron lost through oxidation, another electron must be gained through reduction. This electron transfer is what drives the reaction forward.
In the case of hydrogen peroxide’s decomposition, the electrons lost by hydrogen peroxide in its oxidation are the same electrons gained by oxygen in its reduction. It’s as if nature insists on an unbroken chain of electron exchange.
Redox Reactions in Everyday Life
Redox reactions aren’t confined to the laboratory. They’re happening all around us, shaping our world in ways both subtle and profound. Here are some everyday examples that showcase the power of redox reactions:
Combustion: Fire and Energy Production
One of the most familiar redox reactions is combustion, which is the process of burning a fuel in the presence of oxygen to produce heat and light. This reaction is a vital source of energy in our daily lives, powering engines, heating our homes, and even cooking our food.
The combustion of hydrocarbons, such as methane (CH4), is a classic example:
CH4 + 2O2 → CO2 + 2H2O
In this reaction, methane (the primary component of natural gas) is oxidized to produce carbon dioxide (CO2) and water (H2O). The release of energy during combustion makes it a cornerstone of our modern industrialized society.
Battery-Powered Devices: Portable Energy
Batteries are ubiquitous in our lives, powering everything from smartphones to electric vehicles. At the heart of a battery’s operation is a redox reaction that allows for the controlled release of electrical energy.
In a typical alkaline battery, zinc undergoes oxidation, releasing electrons:
Zn → Zn^2+ + 2e^-
These released electrons travel through an external circuit, providing power to the connected device. Meanwhile, manganese dioxide (MnO2) undergoes reduction:
2MnO2 + 2H2O + 2e^- → 2MnO(OH) + 2OH^-
The flow of electrons from the zinc to manganese dioxide creates an electrical current. As long as the redox reactions continue, the battery supplies electrical energy.
Food Digestion: Extracting Energy from Nutrients
Inside our bodies, a complex series of redox reactions is responsible for extracting energy from the food we eat. Cellular respiration, a crucial metabolic process, involves the oxidation of glucose and the reduction of oxygen to produce adenosine triphosphate (ATP), the molecule that stores and transfers energy within cells.
The overall reaction for cellular respiration can be summarized as follows:
C6H12O6 + 6O2 → 6CO2 + 6H2O + ATP
In this redox reaction, glucose is oxidized to carbon dioxide and water, while oxygen is reduced to water. The energy released during this process is used to fuel various cellular activities, allowing us to perform everyday tasks.
Corrosion: The Slow Redox Erosion
While combustion is a rapid and noticeable form of oxidation, corrosion is its slow and often insidious counterpart. Corrosion involves the gradual degradation of materials, particularly metals, due to exposure to environmental factors, such as moisture and oxygen.
The rusting of iron, as mentioned earlier, is a classic example of corrosion:
4Fe + 3O2 + 6H2O → 4Fe(OH)3
In this redox reaction, iron undergoes oxidation, transforming into iron ions (Fe^3+), while oxygen is reduced to hydroxide ions (OH^-). The formation of rust weakens the metal’s structure over time, leading to structural damage and economic losses.
Environmental Cleanup: Bioremediation
Redox reactions also play a pivotal role in environmental cleanup efforts, particularly in the field of bioremediation. Bioremediation is the use of microorganisms to reduce or eliminate pollutants in soil and groundwater.
One example of bioremediation involves the cleanup of sites contaminated with chlorinated solvents like trichloroethylene (TCE). Certain bacteria, such as Dehalococcoides, are capable of performing reductive dechlorination, a process that reduces TCE to less harmful compounds, ultimately leading to the production of ethene (C2H4) and chloride ions (Cl^-).
The overall reaction for the bioremediation of TCE can be summarized as follows:
3C2HCl3 + 2H2O → 2C2H4 + 6HCl
In this redox reaction, TCE undergoes reduction, while water serves as the electron donor. The result is the transformation of a highly toxic pollutant into less harmful substances.
Wrapping Up
Redox reactions are the dynamic heartbeats of chemistry, driving countless transformations in the world around us. Whether they’re generating energy, powering devices, or breaking down pollutants, these reactions illustrate the intricate dance between oxidation and reduction. As we navigate our daily lives, it’s worth recognizing the role that redox reactions play in shaping the world and enabling our modern way of life. From the flames of combustion to the silent work of corrosion, redox reactions are at the core of our chemical existence, constantly balancing the books of electrons in a never-ending cosmic ledger.
FAQs
Oxidation is a chemical process where an atom, ion, or molecule loses one or more electrons, resulting in an increase in its oxidation state or oxidation number. It often involves the addition of oxygen or the removal of hydrogen.
Reduction is a chemical process where an atom, ion, or molecule gains one or more electrons, leading to a decrease in its oxidation state or oxidation number. It can involve the addition of hydrogen or the removal of oxygen.
Oxidation and reduction are two sides of the same coin and are often interconnected in redox (reduction-oxidation) reactions. In these reactions, one substance undergoes oxidation by losing electrons, while another undergoes reduction by gaining those electrons.
Common examples of oxidation include the rusting of iron, the combustion of fuels, and the spoilage of food when exposed to air.
Certainly! Reduction processes are involved in photosynthesis, battery operation, bioremediation of pollutants, and the use of antioxidants in food preservation.
Oxidation is typically represented with a positive sign (+) for the change in oxidation state, while reduction is often represented with a negative sign (-) for the change in oxidation state.
Oxidation is essential for breaking down nutrients to produce energy in living organisms, while reduction is crucial for building complex molecules and storing energy.
In electrochemical cells, oxidation occurs at the anode, where electrons are lost, while reduction occurs at the cathode, where electrons are gained. These processes generate electrical currents.
Oxidation numbers are assigned to atoms in compounds to track changes in electron distribution during oxidation and reduction. An increase in oxidation number signifies oxidation, while a decrease signifies reduction.
You can explore detailed explanations and examples in the blog post titled “Differences Between Oxidation vs Reduction.”
Read More:
Contents