| Characteristic | Iodine (I2) | Iodide (I-) |
|---|---|---|
| Chemical Formula | I2 | I- |
| Molecular/Ionic Nature | Diatomic Molecule | Ion |
| Charge | Neutral (0) | Negative (-1) |
| Physical State at Room Temperature | Solid (sublimable) | Solid (non-sublimable) |
| Color | Dark violet-black crystals | White crystalline solid |
| Odor | Pungent | Odorless |
| Solubility in Water | Sparingly soluble | Highly soluble |
| Solubility in Organic Solvents | More soluble | Less soluble |
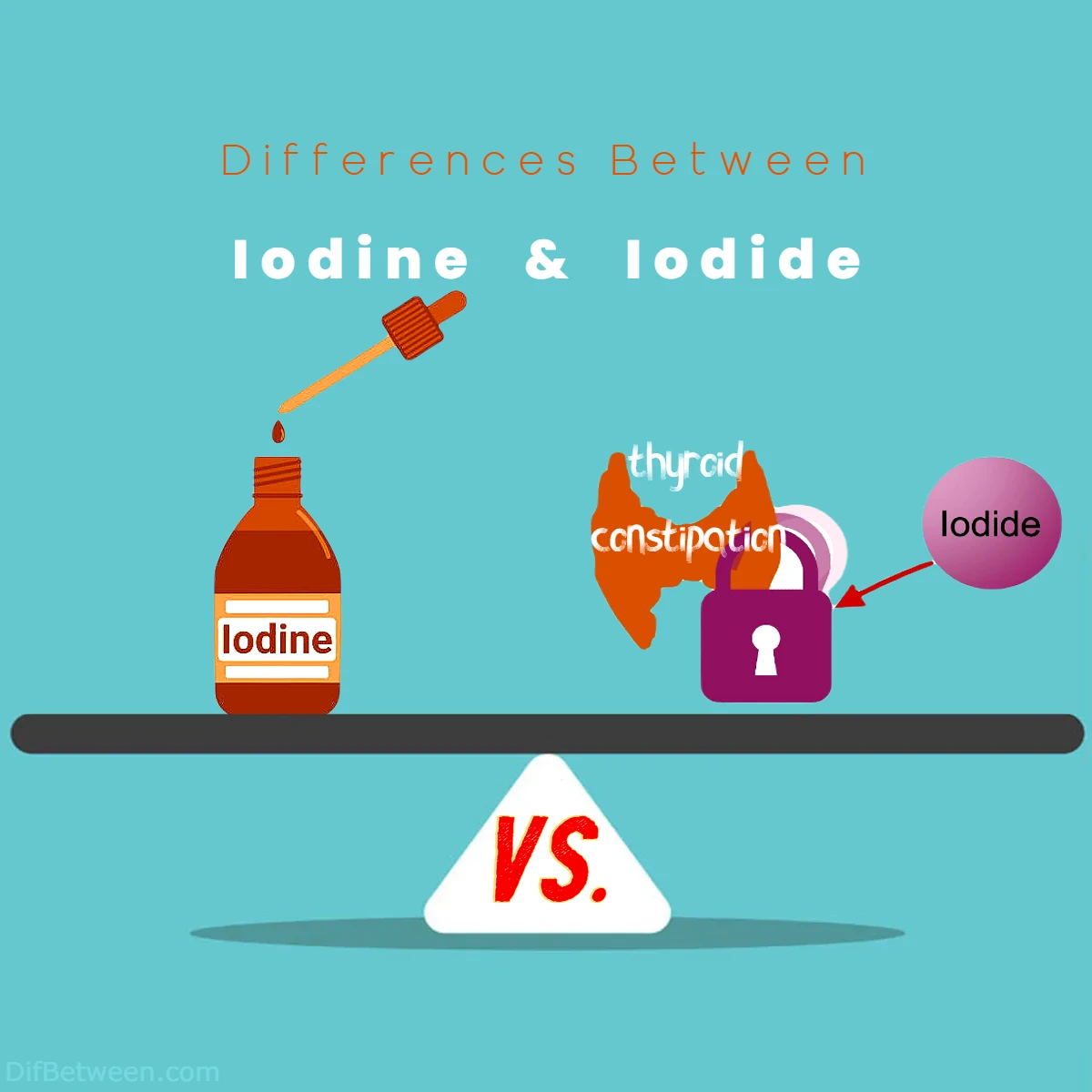
| Role in Thyroid Health | Essential for thyroid hormones | – |
| Use in Disinfection and Cleaning | Commonly used as an antiseptic | – |
| Use in Chemistry Reactions | Used in various chemical reactions | Used in redox and precipitation reactions |
| Use in Microscopy | Used for staining in microscopy | – |
| Use in Nuclear Medicine | Used in diagnostic imaging and treatment | – |
| Use in Pharmaceutical Formulations | – | Used in cough syrups and respiratory medications |
| Use in Radiation Detection | – | Used in scintillation detectors |
| Environmental Impact | Released into the atmosphere and cycles naturally | Present in seawater and plays a role in marine ecosystems |
| Analytical Chemistry Applications | Used in iodometric titrations and iodine-starch tests | Used in redox titrations, complexometric titrations, and precipitation reactions |
| Safety Concerns | Toxic if ingested or inhaled in large amounts, potential for allergic reactions | Generally safe but can impact thyroid function if consumed excessively, potential for allergic reactions |
| Dietary Supplementation | – | Used as potassium iodide supplements in iodine-deficient regions |
To fully grasp the intricate tapestry of differences between iodine and iodide, continue reading to the end of this blog. We’ll delve into their chemical compositions, physical properties, roles in thyroid health, environmental impacts, and much more. Whether you’re a science enthusiast, a healthcare seeker, or simply a curious soul eager to expand your knowledge, understanding the nuances of iodine and iodide will empower you to make informed choices in your daily life.
Differences Between Iodine and Iodide
The main differences between iodine and iodide lie in their chemical nature and properties. Iodine exists as a diatomic molecule, I2, in a solid state with a distinctive violet-black color and a pungent odor, whereas iodide is an ion, I-, found in various salts, typically appearing as white crystalline solids and lacking any strong odor. Iodine is less reactive and mainly used in health for thyroid hormone production and as an antiseptic, while iodide is more reactive, playing crucial roles in pharmaceuticals, analytical chemistry, and radiation protection. These disparities make iodine and iodide distinct yet essential entities in the worlds of science, health, and industry.
Understanding the Basics
Before diving into the nitty-gritty details, it’s essential to establish a fundamental understanding of what iodine and iodide actually are.
Iodine: The Elemental Wonder
Iodine is a chemical element represented by the symbol ‘I’ on the periodic table. It belongs to the halogen group, which includes fluorine, chlorine, bromine, and astatine. Iodine exists as a diatomic molecule, meaning it consists of two iodine atoms bonded together (I2). In its natural state, iodine typically appears as a shiny, violet-black solid with a distinct odor.
Iodine is relatively rare in Earth’s crust, occurring naturally in various forms, such as iodide salts and organoiodine compounds. It is an essential trace element for humans, primarily because of its crucial role in the production of thyroid hormones, which are essential for regulating metabolism and maintaining overall health.
Iodide: The Ionic Partner
Iodide, on the other hand, is the ionic form of iodine. It is essentially iodine that has gained or lost electrons to achieve a stable electronic configuration. Iodide ions are represented as ‘I-‘ because they have an extra electron compared to the neutral iodine atom.
Iodide ions are commonly found in nature, often in the form of salts. Some well-known iodide salts include potassium iodide (KI) and sodium iodide (NaI). These salts are widely used in various industrial, medical, and dietary applications.
Now that we’ve established the basics let’s delve deeper into the differences between these two intriguing chemical entities.
Chemical Composition
One of the primary distinctions between iodine and iodide lies in their chemical composition.
Iodine’s Molecular Structure
As mentioned earlier, iodine exists as a diatomic molecule with the chemical formula I2. This means that it consists of two iodine atoms bonded together through a covalent bond. The two iodine atoms share electrons to form this stable molecule.
Iodide’s Ionic Structure
Iodide, on the other hand, is an ion. It carries a single negative charge, denoted as I-, indicating that it has gained one extra electron to achieve stability. This extra electron is responsible for the negative charge and gives iodide its ionic character.
The table below summarizes the key differences in the chemical composition of iodine and iodide:
| Characteristic | Iodine (I2) | Iodide (I-) |
|---|---|---|
| Chemical Formula | I2 | I- |
| Molecular/Ionic Nature | Diatomic Molecule | Ion |
| Charge | Neutral (0) | Negative (-1) |
Physical Properties: Distinctive Traits
Another set of disparities between iodine and iodide can be observed in their physical properties, such as appearance, state at room temperature, and odor.
Iodine’s Physical Properties
- Appearance: Iodine typically presents itself as shiny, dark violet-black crystals with a metallic luster.
- State at Room Temperature: At standard room temperature and pressure (about 25°C or 77°F), iodine exists in a solid state. However, it undergoes sublimation, meaning it can transition directly from a solid to a gas without passing through a liquid phase.
- Odor: Iodine has a distinct, pungent odor that is often described as resembling that of chlorine.
Iodide’s Physical Properties
- Appearance: Iodide salts are typically white crystalline solids. The appearance can vary slightly depending on the specific salt and its purity.
- State at Room Temperature: Iodide salts are stable solids at room temperature and do not undergo sublimation.
- Odor: Iodide salts are odorless.
Here’s a summarized comparison of the physical properties of iodine and iodide:
| Property | Iodine (I2) | Iodide (I-) salts |
|---|---|---|
| Appearance | Violet-black crystals | White crystalline solids |
| State at Room Temp. | Solid (sublimable) | Solid (non-sublimable) |
| Odor | Pungent | Odorless |
Chemical Reactivity
The chemical reactivity of iodine and iodide is significantly different due to their distinct molecular and ionic natures.
Iodine’s Chemical Reactivity
- Iodine as a Diatomic Molecule: Iodine molecules (I2) are relatively stable and less reactive compared to many other halogens, such as chlorine and fluorine. They do not readily react with most substances at room temperature.
- Reactivity with Metals: Iodine can react with certain metals, especially when heated, to form metal iodides. For example, it reacts vigorously with sodium to produce sodium iodide:Copy code
2Na + I2 → 2NaI - Iodine’s Oxidizing Properties: Iodine can act as a weak oxidizing agent under specific conditions, particularly in the presence of reducing agents.
Iodide’s Chemical Reactivity
- Ionic Character: Iodide ions (I-) are much more reactive than neutral iodine molecules. They readily participate in various chemical reactions, especially in aqueous solutions.
- Formation of Iodine: Iodide ions can be oxidized to form iodine in the presence of strong oxidizing agents. This reaction is commonly used in analytical chemistry to detect the presence of iodide ions.
2I- + Cl2 → 2Cl- + I2 - Precipitation Reactions: Iodide ions can form insoluble salts with certain cations, leading to precipitation reactions. For example, mixing iodide ions with lead(II) ions results in the formation of bright yellow lead iodide (PbI2), which is sparingly soluble in water.
2KI + Pb(NO3)2 → 2KNO3 + PbI2 (precipitate)
The contrasting reactivity of iodine and iodide stems from their molecular and ionic natures, making them suitable for different chemical applications.
Solubility: Dissolving Behavior
Solubility is a vital aspect of chemistry, as it determines how substances interact with solvents, particularly water. The solubility of iodine and iodide differs significantly.
Iodine’s Solubility
- Water Solubility: Iodine is sparingly soluble in water. It dissolves to a limited extent, forming a brownish solution due to the formation of a complex called the triiodide ion (I3-).cssCopy code
I2 + I- ⇌ I3- - Organic Solvents: Iodine is more soluble in organic solvents like chloroform and carbon tetrachloride than in water. This property makes it useful for various chemical reactions and tests, such as the famous iodine test for the presence of starch.
Iodide’s Solubility
- Water Solubility: Iodide salts, such as potassium iodide (KI) and sodium iodide (NaI), are highly soluble in water. When these salts are added to water, they readily dissociate into iodide ions (I-) and the respective cations (K+ or Na+).mathematicaCopy code
KI → K+ + I- NaI → Na+ + I- - Solubility in Organic Solvents: Iodide salts are generally not soluble in organic solvents like chloroform or ether to the same extent as iodine.
The solubility differences between iodine and iodide are crucial for various applications, including the preparation of iodine solutions and pharmaceutical formulations.
Uses and Applications
Iodine and iodide find diverse applications across various fields, thanks to their unique properties and reactivities. Let’s explore their uses in more detail.
Applications of Iodine
- Thyroid Function: Iodine is an essential component for the synthesis of thyroid hormones, thyroxine (T4), and triiodothyronine (T3). A deficiency in iodine can lead to thyroid disorders like goiter and hypothyroidism. Iodized salt, which contains potassium iodide, is a common method of ensuring sufficient iodine intake in diets.
- Disinfectant: Iodine-based compounds, such as iodine tincture and povidone-iodine (Betadine), are used as antiseptics and disinfectants for cleaning wounds and skin before surgical procedures.
- Chemical Reagent: Iodine is employed as a reagent in various chemical reactions, including the iodine test for starch, redox titrations, and organic synthesis.
- Dye: Iodine vapor can be used to stain certain materials, such as cell samples and biological specimens, for microscopy.
- Radioactive Iodine: Radioactive iodine isotopes, such as iodine-131, are used in nuclear medicine for diagnostic imaging and the treatment of thyroid disorders and certain cancers.
Applications of Iodide
- Pharmaceuticals: Iodide salts, particularly potassium iodide (KI), are used in pharmaceutical preparations, such as cough syrups, to treat respiratory conditions like chronic obstructive pulmonary disease (COPD).
- Radiation Shielding: Sodium iodide (NaI) crystals doped with thallium are commonly used as scintillation detectors in radiation detection equipment, such as gamma-ray spectrometers.
- Photography: Silver iodide (AgI) is used in the photographic industry to create light-sensitive emulsions in photographic films and papers.
- Nuclear Reactors: Iodide compounds are employed in nuclear reactors to help control the reactivity of nuclear fuel.
- Analytical Chemistry: Iodide ions are used in analytical chemistry techniques, including titrations and colorimetric assays.
Health Implications
Both iodine and iodide have profound implications for human health, but their roles differ due to their distinct forms.
Iodine and Thyroid Health
Iodine is crucial for the proper functioning of the thyroid gland, which plays a central role in regulating metabolism. Here’s how iodine relates to thyroid health:
- Thyroid Hormone Production: The thyroid gland synthesizes thyroid hormones, T4 and T3, by incorporating iodine into their molecular structure. These hormones are essential for controlling the body’s metabolic rate, energy production, and overall growth and development.
- Iodine Deficiency: Insufficient iodine intake can lead to thyroid disorders, including goiter (an enlarged thyroid gland) and hypothyroidism (underactive thyroid). These conditions can result in fatigue, weight gain, and other health issues.
- Iodized Salt: The addition of iodine as potassium iodide to table salt (iodized salt) is a common public health measure to prevent iodine deficiency disorders. Regular consumption of iodized salt helps ensure an adequate intake of iodine.
Iodide in Pharmaceuticals
Iodide salts, particularly potassium iodide (KI), have medical applications as well:
- Respiratory Conditions: Potassium iodide is sometimes used to treat respiratory conditions like chronic obstructive pulmonary disease (COPD) and asthma. It can help reduce mucus production and alleviate symptoms during exacerbations.
- Radioprotection: In the event of a nuclear radiation emergency, potassium iodide tablets are distributed to people living in affected areas. Consuming these tablets can saturate the thyroid gland with non-radioactive iodide, reducing the risk of absorbing radioactive iodine isotopes.
Safety Considerations
While both iodine and iodide have important applications, they also come with safety considerations and potential health risks.
Safety Concerns with Iodine
- Toxicity: Pure elemental iodine (I2) is toxic when ingested or inhaled in large amounts. It can cause irritation of the digestive and respiratory tract, leading to symptoms such as nausea, vomiting, and coughing.
- Allergic Reactions: Some individuals may be sensitive or allergic to iodine-based compounds. Allergic reactions can range from mild skin rashes to severe anaphylaxis.
- Thyroid Dysfunction: Excessive iodine intake, especially from dietary supplements, can lead to thyroid dysfunction, including hyperthyroidism (overactive thyroid).
Safety Considerations with Iodide
- Thyroid Effects: Iodide salts, such as potassium iodide, are generally safe when used as directed. However, excessive iodide intake can also affect thyroid function, potentially leading to thyroid disorders.
- Allergic Reactions: Like iodine, iodide compounds can cause allergic reactions in sensitive individuals.
- Drug Interactions: Iodide medications may interact with other drugs or substances, so it’s essential to inform healthcare providers about all medications and supplements being taken.
Environmental Impact
Beyond their roles in health and chemistry, iodine and iodide also have implications for the environment. Here, we explore how these substances can impact our surroundings.
Iodine in the Environment
- Iodine Cycle: Iodine exists in a complex cycle within the environment. It is released into the atmosphere from the oceans through natural processes like wave action and wind. Once in the atmosphere, iodine can participate in various chemical reactions and eventually return to the Earth’s surface through rainfall.
- Ecological Significance: Iodine is essential for marine life, as it is taken up by seaweeds and phytoplankton. These organisms serve as the primary sources of iodine for marine ecosystems. Additionally, iodine can act as a natural antioxidant, protecting marine organisms from oxidative stress.
- Radioactive Iodine: One environmental concern related to iodine is the release of radioactive iodine isotopes, such as iodine-131, from nuclear accidents or nuclear waste. Radioactive iodine can contaminate soil and water, posing health risks to both humans and wildlife.
Iodide in the Environment
- Natural Abundance: Iodide ions are naturally present in seawater in relatively high concentrations. This is due to the constant release of iodine from the Earth’s crust into the oceans.
- Iodine Deficiency in Soil: In some regions, particularly those far from the coast, the soil may be deficient in iodine. This deficiency can affect the iodine content of crops grown in these areas, potentially leading to iodine deficiency disorders in humans who consume these crops.
- Environmental Fate: Iodide ions are relatively stable in the environment and do not undergo significant chemical changes in natural conditions. However, they can be subject to biological transformations by certain microorganisms.
Analytical Techniques
In the field of analytical chemistry, both iodine and iodide play crucial roles in various techniques and tests. Here, we explore their significance in this realm.
Iodine in Analytical Chemistry
- Iodometric Titrations: Iodine is commonly used as a titrant in iodometric titrations, a type of redox titration. This technique involves the use of iodine to determine the concentration of reducing agents or analytes. For example, it can be used to determine the concentration of vitamin C (ascorbic acid) in a solution.
- Iodine-Starch Test: The iodine-starch test is a qualitative test for the presence of starch in a solution. Iodine reacts with starch to form a blue-black complex. This test is widely used in food science and biology laboratories to detect the presence of starch in various samples.
- Colorimetric Assays: Iodine is used in colorimetric assays to measure the concentration of certain analytes based on the color change that occurs during the reaction with iodine. One example is the determination of arsenic concentration in water using the Gutzeit method.
Iodide in Analytical Chemistry
- Redox Titrations: Iodide ions (I-) are frequently used as reducing agents in redox titrations. They can reduce various oxidizing agents, and the reaction is monitored using a suitable indicator or potentiometrically. For instance, iodide ions can reduce permanganate ions (MnO4-) in a titration to determine the concentration of analytes like hydrogen peroxide (H2O2).
- Precipitation Reactions: Iodide ions are involved in precipitation reactions, leading to the formation of sparingly soluble salts. This property can be utilized in qualitative analysis to identify the presence of specific cations by observing the formation of characteristic precipitates.
- Complexometric Titrations: Iodide ions can serve as complexing agents in complexometric titrations, where they form complexes with certain metal ions. These titrations are used to determine the concentration of metal ions in a solution.
Iodine vs. Iodide Supplements: Dietary Considerations
For individuals looking to supplement their iodine intake, understanding the differences between iodine and iodide supplements is crucial.
Iodine Supplements
- Iodine Sources: Iodine supplements typically provide iodine in the form of potassium iodide (KI) or potassium iodate (KIO3). These supplements are designed to provide a consistent source of iodine for individuals who may not obtain sufficient iodine from their diets.
- Thyroid Health: Iodine supplements are primarily used to support thyroid health and prevent iodine deficiency disorders. They are available in various forms, including tablets, capsules, and liquid solutions.
- Caution: It’s important to use iodine supplements as directed by a healthcare professional. Excessive iodine intake from supplements can lead to thyroid dysfunction.
Iodide Supplements
- Iodide Sources: Iodide supplements, specifically potassium iodide (KI), are sometimes used in emergency preparedness kits. They are intended for use in the event of nuclear radiation emergencies to protect the thyroid gland from radioactive iodine absorption.
- Nuclear Emergencies: In cases where there is a risk of exposure to radioactive iodine isotopes (e.g., during a nuclear accident), potassium iodide tablets can be distributed to individuals in affected areas as a preventive measure.
- Proper Usage: The use of iodide supplements for radiation protection should be guided by local authorities and healthcare professionals during emergency situations.
Iodine or Iodide: Which One is Right Choose for You?
Iodine and iodide are two chemical entities that share a close connection but serve distinct purposes and offer unique benefits. When considering which one is right for you, it’s essential to understand their differences and the specific contexts in which each is most appropriate.
When to Choose Iodine:
- Thyroid Health: If you’re looking to support your thyroid health, iodine is the way to go. It is an essential element for the production of thyroid hormones, which play a critical role in regulating metabolism and overall well-being.
- Disinfection and Cleaning: Iodine-based compounds, such as iodine tincture and povidone-iodine, are excellent choices for disinfection and wound cleaning. They have antiseptic properties and are commonly used in healthcare settings.
- Chemical Reactions: If you’re a chemistry enthusiast or involved in laboratory work, iodine serves as a versatile reagent in various chemical reactions, from redox titrations to organic synthesis.
- Staining in Microscopy: Iodine vapor is used in microscopy to stain biological specimens, making them more visible for examination.
- Radioactive Iodine: In medical diagnostics and treatment, radioactive iodine isotopes like iodine-131 are used for imaging and therapy in conditions like thyroid cancer.
When to Choose Iodide:
- Respiratory Conditions: Iodide salts, especially potassium iodide (KI), are used in pharmaceuticals to treat respiratory conditions like chronic obstructive pulmonary disease (COPD) and asthma. They can help reduce mucus production and alleviate symptoms.
- Radiation Protection: In situations where there is a risk of exposure to radioactive iodine isotopes (e.g., during a nuclear accident), potassium iodide tablets are crucial for protecting the thyroid gland from absorbing radioactive iodine.
- Analytical Chemistry: If you’re involved in analytical chemistry, iodide ions (I-) are valuable for redox titrations, complexometric titrations, and precipitation reactions.
- Colorimetric Assays: Iodide can be used in colorimetric assays to measure the concentration of specific analytes based on color changes during the reaction.
- Environmental Detection: Iodide is used in environmental monitoring and radiation detection equipment, such as scintillation detectors.
- Dietary Supplementation: In areas with iodine-deficient soil, iodide supplements like potassium iodide may be necessary to prevent iodine deficiency disorders.
In essence, the choice between iodine and iodide depends on your specific needs and objectives. If you’re aiming to support your thyroid health, disinfect wounds, or explore the wonders of chemistry, iodine is your ally. On the other hand, if you’re dealing with respiratory conditions, need radiation protection, or are immersed in analytical chemistry, iodide is the partner you’re looking for. Understanding their differences empowers you to make informed decisions in both your daily life and professional endeavors.
FAQs
The fundamental difference between iodine and iodide is their chemical nature. Iodine (I2) is a diatomic molecule, existing as a shiny, violet-black solid with a pungent odor. Iodide (I-) is an ion, carrying a single negative charge, and is typically found in the form of salts, appearing as white crystalline solids without a strong odor.
Iodine is essential for thyroid health as it is a key component in the production of thyroid hormones, crucial for regulating metabolism. Iodide, on the other hand, is indirectly related to thyroid health because it’s a source of iodine when consumed as part of iodide salts like potassium iodide, which helps prevent iodine deficiency disorders.
Certainly! Iodine is sparingly soluble in water and more soluble in organic solvents like chloroform. In contrast, iodide salts, such as potassium iodide and sodium iodide, are highly soluble in water but generally less soluble in organic solvents.
Iodine can be toxic when ingested or inhaled in large amounts and may cause allergic reactions in some individuals. Excessive iodine intake can also affect thyroid function. Iodide, while generally safe, can impact thyroid function when consumed excessively and may also cause allergic reactions.
Iodine is used in iodometric titrations and iodine-starch tests, whereas iodide is employed in redox titrations, complexometric titrations, and precipitation reactions in analytical chemistry.
Iodine cycles naturally in the environment, with release into the atmosphere from oceans. It plays a role in marine ecosystems and can be involved in the environmental fate of certain compounds. Iodide is naturally present in seawater and is crucial for marine life, particularly in coastal regions.
Iodine is used for disinfection, wound cleaning, and thyroid health. Iodide, in the form of potassium iodide, is used in pharmaceuticals to treat respiratory conditions and for radiation protection in the event of nuclear emergencies.
Iodine supplements provide a consistent source of iodine for thyroid health, while iodide supplements, like potassium iodide, may be used in regions with iodine-deficient soil to prevent iodine deficiency disorders.
Iodine isotopes, including iodine-131, are used in nuclear medicine for diagnostic imaging and the treatment of thyroid disorders and certain cancers. Iodide, specifically potassium iodide, is used in radiation protection during nuclear emergencies.
Iodine has a pungent odor and can impart a metallic taste when ingested, whereas iodide salts are typically odorless and do not significantly affect taste or smell.
Read More:
Contents