| Property | Ammonia | Ammonium Hydroxide |
|---|---|---|
| Chemical Formula | NH3 | NH4OH (NH3 in solution) |
| State at Room Temp. | Gas | Liquid Solution |
| Odor | Pungent, suffocating | Pungent (similar to ammonia) |
| Density | Less dense than air | Higher density than ammonia |
| Solubility | Highly soluble in water | Fully miscible in water |
| Basicity | Weak base | Weak base |
| Reactivity | Forms ammonium salts | Used in various chemical processes |
| Volatility | Highly volatile | Less volatile due to solution |
| Concentration | N/A | Varies (e.g., 5%, 10%, 30%) |
| pH | N/A | Basic (pH > 7) |
| Production | Haber-Bosch process | Dissolving ammonia in water |
| Environmental Impact | Air and water pollution | Risk of ammonia contamination |
| Common Uses | Cleaning, refrigeration, | Cleaning, laboratories, |
| agriculture, pH control | food industry, water treatment | |
| Safety Considerations | Inhalation hazard, skin | Inhalation hazard, skin and |
| and eye irritation | eye irritation, corrosive |
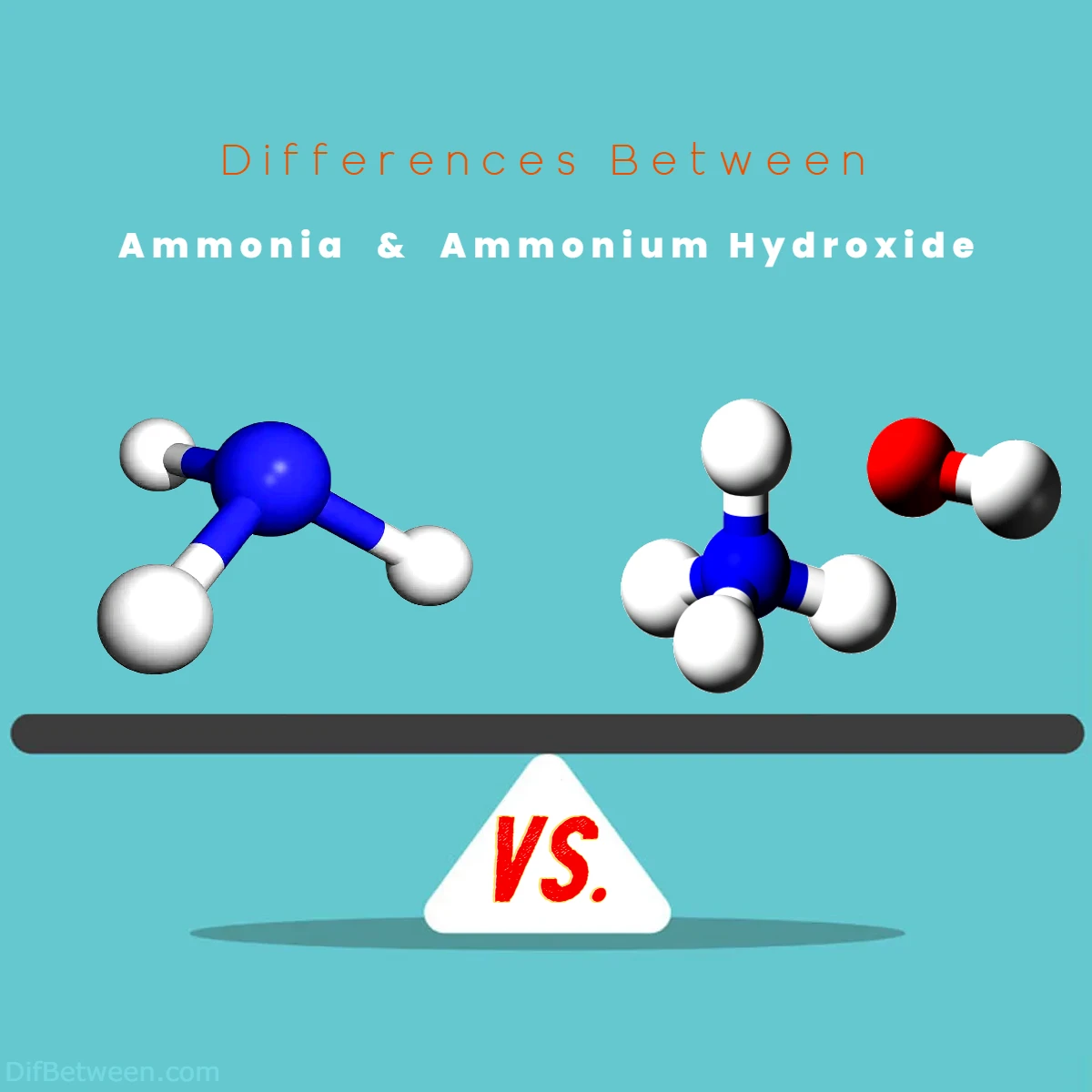
Ammonia, represented by the chemical formula NH3, is a colorless gas with a rather notorious pungent odor. It’s a versatile chemical, finding its way into household cleaners, refrigeration systems, and even the vast fields of agriculture. On the other hand, ammonium hydroxide, often lurking under the radar, is a liquid solution derived from ammonia by dissolving it in water. While it shares that unmistakable ammonia scent, its uses span from laboratories to bakeries, making it a key ingredient in various aspects of our daily lives.
Differences Between Ammonia and Ammonium Hydroxide
The main differences between Ammonia and Ammonium Hydroxide lie in their physical states and composition. Ammonia, with the chemical formula NH3, exists as a colorless gas, known for its pungent odor. In contrast, Ammonium Hydroxide, represented as NH4OH, is a liquid solution formed by dissolving ammonia in water, retaining the ammonia scent but presenting itself in a different state. This distinction in physical properties leads to varied applications and safety considerations for these two chemicals, making it essential to discern their differences for appropriate usage.
1. Chemical Composition
Ammonia
Ammonia, represented by the chemical formula NH3, is a compound composed of one nitrogen atom (N) bonded to three hydrogen atoms (H). This molecular structure gives ammonia its distinctive properties and characteristics.
Ammonium Hydroxide
Ammonium hydroxide, on the other hand, is not a pure compound like ammonia. It is an aqueous solution of ammonia gas (NH3) dissolved in water (H2O). This solution contains both ammonia (NH3) and water (H2O) molecules. In chemical terms, it can be represented as NH4OH, indicating the presence of the ammonium ion (NH4+) and hydroxide ion (OH-) in solution.
The key difference in chemical composition between ammonia and ammonium hydroxide lies in their physical state and the presence of water in the latter.
2. Physical Properties
Ammonia
State: Ammonia is a colorless gas at room temperature and standard pressure (25°C and 1 atmosphere).
Odor: One of the most distinctive features of ammonia is its pungent, suffocating odor, which is often described as smelling like household cleaning products.
Density: Ammonia is less dense than air, which is why it can rise in the atmosphere.
Solubility: Ammonia is highly soluble in water, forming ammonium hydroxide when dissolved.
Ammonium Hydroxide
State: Ammonium hydroxide is a liquid solution.
Odor: It shares the same pungent odor as pure ammonia due to the presence of ammonia gas in solution.
Concentration: The concentration of ammonia in ammonium hydroxide solutions can vary, depending on the intended application. Common concentrations include 5%, 10%, and 30%, with the remaining portion being water.
pH: Ammonium hydroxide solutions are basic, meaning they have a pH greater than 7.
In summary, ammonia exists as a gas, while ammonium hydroxide is a liquid solution containing ammonia dissolved in water. The odor and properties of ammonium hydroxide are largely influenced by the presence of ammonia gas.
3. Chemical Properties
Ammonia
Basicity: Ammonia is a weak base. It can accept a proton (H+) from a water molecule, forming ammonium ions (NH4+) and hydroxide ions (OH-).
Reactivity: Ammonia can react with various substances, including acids, to form ammonium salts. It can also participate in reactions such as the synthesis of ammonium nitrate, which is essential in the fertilizer industry.
Volatility: Ammonia is highly volatile and can readily evaporate into the air.
Ammonium Hydroxide
Basicity: Ammonium hydroxide solutions are also weak bases. They can donate hydroxide ions (OH-) to acidic substances.
Reactivity: These solutions can be used in a variety of chemical processes, such as metal cleaning and as a reagent in laboratories.
Stability: Unlike pure ammonia, ammonium hydroxide solutions are relatively stable and do not evaporate as readily.
While both ammonia and ammonium hydroxide exhibit basic properties, their chemical reactivity and stability differ due to the presence of water in the latter.
4. Common Uses
Ammonia
Household Cleaning: Ammonia is commonly found in household cleaning products, such as glass cleaners and floor cleaners, due to its ability to dissolve grease and stains.
Refrigeration: Ammonia is used as a refrigerant in industrial refrigeration systems, where its low boiling point makes it effective for cooling.
Agriculture: It is a key component in the production of nitrogen-based fertilizers like ammonium nitrate, which provides essential nutrients to plants.
Cleaning and Degreasing: In industrial settings, ammonia is used for cleaning and degreasing equipment and machinery.
pH Control: Ammonia can be used to adjust the pH of solutions in various applications, including wastewater treatment.
Ammonium Hydroxide
Cleaning: Ammonium hydroxide solutions are used in household cleaning products, similar to ammonia, for their degreasing and stain-removing properties.
Laboratories: They serve as reagents in laboratory experiments, especially in analytical chemistry and titration processes.
Food Industry: Ammonium hydroxide is used as a food additive (E527) to adjust the pH of certain food products.
Textile Industry: It is employed in the textile industry for dyeing and printing fabrics.
Water Treatment: Ammonium hydroxide is utilized in water treatment processes to control pH and remove contaminants.
In summary, both ammonia and ammonium hydroxide find applications in cleaning, industrial processes, and pH control. However, the liquid form of ammonium hydroxide makes it more versatile in some applications, such as laboratory work and the food industry.
5. Safety Considerations
Ammonia
Inhalation Hazard: Ammonia gas is highly toxic when inhaled and can cause severe respiratory irritation, coughing, and even asphyxiation in high concentrations.
Skin and Eye Irritation: Contact with ammonia can lead to skin and eye irritation, causing redness, itching, and burns.
Flammability: Ammonia is not flammable but can support combustion, posing fire risks when in contact with certain substances.
Storage: It should be stored in well-ventilated areas in tightly sealed containers to prevent leaks.
Ammonium Hydroxide
Inhalation Hazard: Like ammonia gas, ammonium hydroxide solutions can release ammonia vapor, which poses inhalation risks. Adequate ventilation is essential when handling these solutions.
Skin and Eye Irritation: Contact with ammonium hydroxide can cause skin and eye irritation similar to pure ammonia.
Corrosive: Concentrated solutions of ammonium hydroxide can be corrosive to certain metals and materials.
Storage: They should be stored in containers designed for corrosive materials and kept away from incompatible substances.
Both ammonia and ammonium hydroxide require careful handling and storage due to their potential health hazards. Protective equipment such as gloves and goggles should be used when working with these chemicals.
6. Production
Ammonia
Ammonia is primarily produced through the Haber-Bosch process, a chemical reaction that combines nitrogen from the air with hydrogen derived from natural gas or other hydrocarbon sources. This process involves high pressures and temperatures, making it energy-intensive.
Ammonium Hydroxide
Ammonium hydroxide is not produced as a separate chemical; rather, it is prepared by dissolving ammonia gas in water. This process allows for various concentrations of ammonium hydroxide to be easily obtained by controlling the amount of ammonia gas added to the water.
7. Environmental Impact
Ammonia
Air Pollution: The release of ammonia into the atmosphere from industrial and agricultural sources can contribute to air pollution. It can react with other pollutants to form particulate matter and ground-level ozone, both of which have adverse effects on human health and the environment.
Water Pollution: Ammonia runoff from agricultural fields and wastewater treatment plants can contaminate surface water and groundwater. Excessive ammonia levels in aquatic ecosystems can harm aquatic life, leading to oxygen depletion and the formation of “dead zones.”
Ammonium Hydroxide
The environmental impact of ammonium hydroxide is closely tied to that of ammonia since it contains ammonia in solution. The risks associated with ammonium hydroxide are mainly related to spills, leaks, or improper disposal, which can lead to ammonia contamination in the environment.
8. Further Applications
Ammonia
Explosives: Ammonia is used in the manufacture of explosives, such as ammonium nitrate-based explosives.
Pharmaceuticals: It serves as a precursor in the production of various pharmaceuticals and medicines.
Analytical Chemistry: In analytical chemistry, ammonia can be employed as a buffer solution for pH adjustment during experiments and titrations.
Ammonium Hydroxide
Cosmetics: Ammonium hydroxide is used in cosmetics as a pH adjuster in products like hair dyes and skin creams.
Bakeries: It can be used in the baking industry as a leavening agent.
Cleaning Agents: In addition to household cleaning products, ammonium hydroxide is used in industrial cleaning agents and detergents.
9. Regulatory Considerations
Ammonia
Ammonia is subject to various regulations and safety standards due to its potential hazards. Occupational Safety and Health Administration (OSHA) in the United States, for instance, sets permissible exposure limits (PELs) for ammonia in workplaces.
Ammonium Hydroxide
Regulations regarding ammonium hydroxide vary depending on its intended use and concentration. For instance, the U.S. Food and Drug Administration (FDA) regulates its use in the food industry as a food additive.
10. Notable Compounds Related to Ammonia
Ammonium Nitrate
Ammonium nitrate (NH4NO3) is a chemical compound derived from ammonia. It is widely used in agriculture as a high-nitrogen fertilizer and in the production of explosives, particularly in the mining industry.
Ammonium Chloride
Ammonium chloride (NH4Cl) is another compound formed from ammonia. It has various applications, including in the food industry as a food additive, as a flux in soldering, and in dry-cell batteries.
11. Notable Compounds Related to Ammonium Hydroxide
Ammonium Carbonate
Ammonium hydroxide can react with carbon dioxide (CO2) to form ammonium carbonate ((NH4)2CO3). This compound is used in baking as a leavening agent and in smelling salts.
Ammonium Sulfate
Ammonium sulfate ((NH4)2SO4) is formed by reacting ammonium hydroxide with sulfuric acid. It is commonly used in fertilizers and flame retardants.
12. Storage and Handling
Ammonia
Ammonia should be stored in well-ventilated areas in containers designed for ammonia storage. Safety precautions, such as the use of personal protective equipment (PPE) and gas detectors, are essential when handling ammonia.
Ammonium Hydroxide
Ammonium hydroxide solutions should be stored in well-labeled containers away from incompatible substances. Proper ventilation is crucial to prevent the buildup of ammonia vapor in enclosed spaces.
13. Emergency Response
Ammonia
In the event of a leak or spill of ammonia, immediate action should be taken to evacuate the area and call emergency responders. Specialized hazmat teams are often required to handle ammonia emergencies.
Ammonium Hydroxide
Emergency response procedures for ammonium hydroxide spills or leaks are similar to those for ammonia since ammonium hydroxide releases ammonia vapor. Evacuation, ventilation, and contacting appropriate authorities are essential steps.
FAQs
The primary difference lies in their physical state and composition. Ammonia (NH3) exists as a colorless gas with a pungent odor, while ammonium hydroxide (NH4OH) is a liquid solution formed by dissolving ammonia in water, retaining the ammonia scent but presenting itself as a liquid.
No, they are not chemically the same. Ammonia is the gaseous form of the compound, while ammonium hydroxide is a solution containing ammonia dissolved in water. Ammonium hydroxide can release ammonia vapor under certain conditions.
Ammonia is commonly used in household cleaning products, refrigeration systems, agriculture (as a component of fertilizers), and as a pH adjuster in various applications. Ammonium hydroxide is used in cleaning agents, laboratories, the food industry (as a pH adjuster), and water treatment processes.
Yes, both substances have safety considerations. Ammonia gas can be highly toxic when inhaled, leading to respiratory issues, and it can cause skin and eye irritation. Ammonium hydroxide solutions also release ammonia vapor and can irritate the skin and eyes. Proper handling, ventilation, and safety equipment are essential when working with these chemicals.
Ammonia releases can contribute to air pollution and water pollution when not properly managed. It can react with other pollutants to form harmful substances. Ammonium hydroxide, due to its ammonia content, can pose similar environmental risks, especially if spilled or released improperly.
Ammonia is primarily produced through the energy-intensive Haber-Bosch process, which combines nitrogen from the air with hydrogen derived from hydrocarbon sources. Ammonium hydroxide, on the other hand, is simply prepared by dissolving ammonia gas in water to create various concentrations of the solution.
Read More:
Contents
- Differences Between Ammonia and Ammonium Hydroxide
- 1. Chemical Composition
- 2. Physical Properties
- 3. Chemical Properties
- 4. Common Uses
- 5. Safety Considerations
- 6. Production
- 7. Environmental Impact
- 8. Further Applications
- 9. Regulatory Considerations
- 10. Notable Compounds Related to Ammonia
- 11. Notable Compounds Related to Ammonium Hydroxide
- 12. Storage and Handling
- 13. Emergency Response
- FAQs