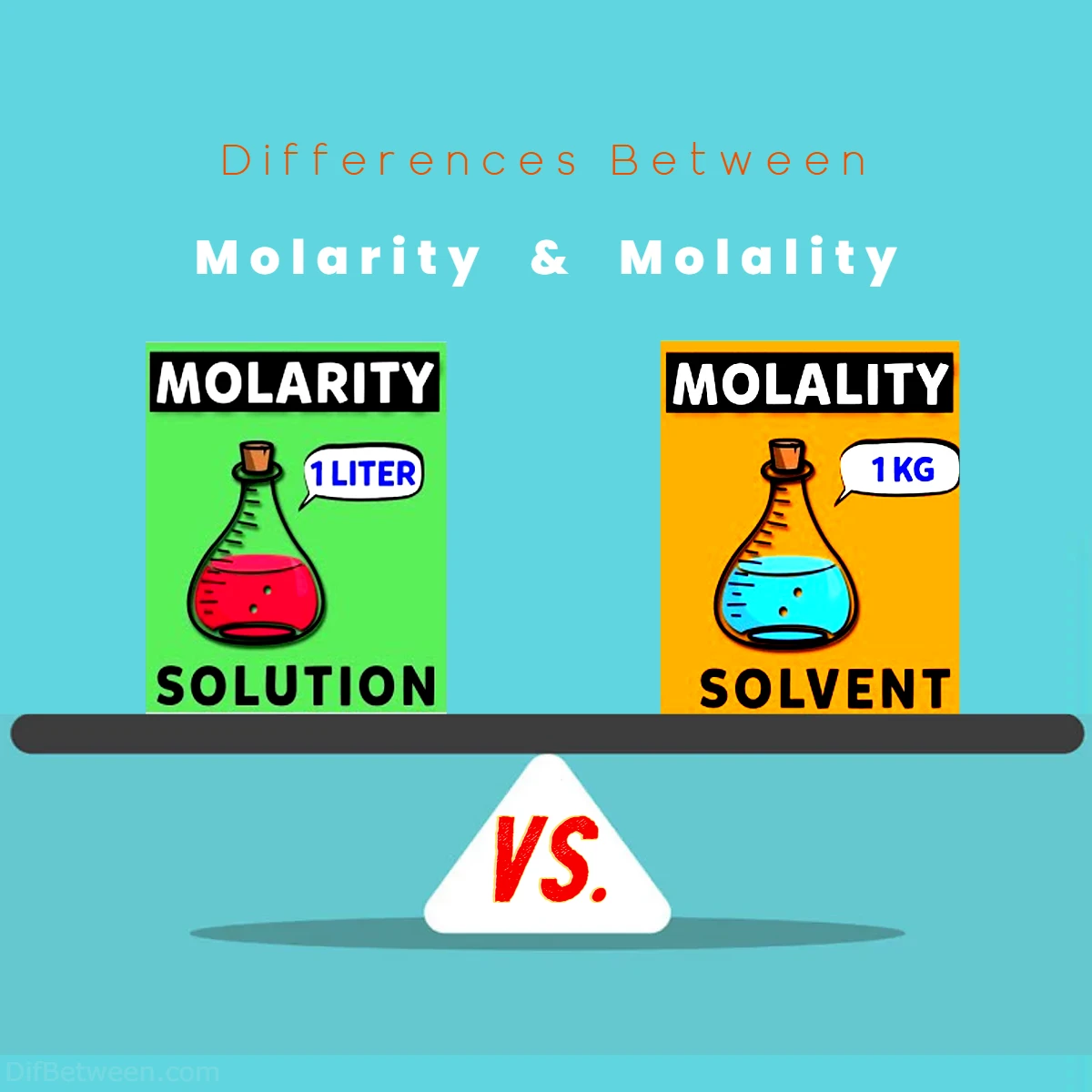
| Aspect | Molarity | Molality |
|---|---|---|
| Definition | Moles of solute per liter of solution | Moles of solute per kilogram of solvent |
| Concentration Measure | Volume-based | Mass-based |
| Symbol | M (mol/L) | m (mol/kg) |
| Temperature Dependence | Temperature-sensitive | Temperature-independent |
| Common Units | mol/L | mol/kg |
| Real-Life Applications | Pharmaceutical manufacturing, food and beverage industry, environmental analysis, general laboratory work | Cryogenics, temperature-sensitive chemical reactions, precise laboratory research |
| Solution Preparation | Focuses on the volume of the solution, molarity determines the concentration based on volume | Focuses on the mass of the solvent, molality emphasizes the mass of the solvent regardless of the solution volume |
| Effects of Temperature | Subject to temperature-induced volume changes, requiring temperature corrections for accuracy | Remains constant with temperature changes, making it suitable for precise experiments under varying temperatures |
| Conversion to/from Molarity | Requires consideration of solution density (�ρ) due to volume dependence | Straightforward conversion, assuming relatively constant density of the solution |
| Role in Colligative Properties | Used in osmotic pressure calculations | Directly related to boiling-point elevation calculations |
Have you ever found yourself knee-deep in the captivating world of chemistry, stirring up solutions and deciphering the mysteries of concentration? If so, you’ve undoubtedly come across two intriguing terms that often make you ponder: Molarity and Molality.
Differences Between Molarity and Molality
The main differences between Molarity and Molality lie in their definitions and practical applications. Molarity, denoted as M, measures the moles of solute per liter of solution and is commonly used in various industries, making it temperature-sensitive due to its reliance on volume. In contrast, Molality, represented as m, measures moles of solute per kilogram of solvent, remaining temperature-independent. Molality is preferred in temperature-sensitive scenarios, such as cryogenics and specific chemical reactions, where maintaining constant concentration regardless of temperature fluctuations is paramount. Understanding these distinctions is crucial for scientists and chemists working with solutions, allowing them to choose the appropriate concentration measure for their unique needs.
1. Definition and Basic Concepts
Molarity:
Molarity, denoted as M, is a measure of the concentration of a solute in a solution. It is defined as the number of moles of solute dissolved in one liter of solution. In other words, molarity tells us how many moles of the solute are present in each liter of the solution.
Molality:
On the other hand, molality, represented as m, is a measure of the concentration of a solute in a solution as well, but it takes a slightly different approach. Molality is defined as the number of moles of solute dissolved in one kilogram of solvent. It focuses on the relationship between the solute and the solvent specifically, regardless of the total solution volume.
2. The Role of Temperature
Molarity:
One of the critical differences between molarity and molality is their dependence on temperature. Molarity is temperature-sensitive. This means that as the temperature of the solution changes, the molarity can also change. This is because molarity is based on the volume of the solution, and temperature can affect the volume.
To account for temperature changes when using molarity, you may need to correct the volume of the solution. This correction is necessary because the volume of a liquid can expand or contract with temperature fluctuations. Scientists often use a reference temperature, such as 25°C (298 K), to calculate molarity accurately.
Molality:
In contrast, molality is temperature-independent. It depends only on the mass of the solvent, which is not significantly affected by temperature changes. This makes molality a preferred choice when working with solutions under varying temperature conditions.
Because molality remains constant regardless of temperature, it is especially useful in situations where precision is crucial, such as in some chemical reactions and laboratory experiments.
Here’s a simple table summarizing the key differences we’ve explored so far:
| Aspect | Molarity | Molality |
|---|---|---|
| Definition | Moles of solute per liter of solution | Moles of solute per kilogram of solvent |
| Temperature Dependence | Temperature-sensitive | Temperature-independent |
3. Application in Real-Life Scenarios
Molarity:
Molarity is commonly used in various real-life situations, especially in industries where precise control of chemical concentrations is crucial. Here are some examples:
A. Pharmaceutical Industry:
In drug manufacturing, pharmaceutical companies need to ensure that the right amount of active ingredients is present in each dosage form, such as tablets or syrups. Molarity helps them determine the concentration of these active ingredients accurately.
B. Food and Beverage Industry:
In the food and beverage industry, molarity is used to measure the concentration of additives, preservatives, and flavorings in products like soft drinks, fruit juices, and sauces. Maintaining the correct molarity ensures consistent product quality.
C. Environmental Monitoring:
Environmental scientists may use molarity to analyze water samples for pollutants or chemicals. By knowing the molarity of a particular pollutant, they can assess the environmental impact and take appropriate measures for remediation.
Molality:
Molality finds its application in specific scenarios where temperature variations play a crucial role. Here are some examples:
A. Cryogenics:
In cryogenic applications, where extremely low temperatures are involved, molality is preferred because it remains constant despite temperature changes. This is essential when working with liquefied gases like liquid nitrogen or helium.
B. Chemical Reactions:
Certain chemical reactions are highly temperature-sensitive. Chemists use molality to ensure that the concentration of reactants remains constant, irrespective of temperature fluctuations, to obtain accurate and reproducible results.
C. Laboratory Research:
In scientific research, especially in fields like chemistry and biochemistry, where precise measurements are vital, molality is a preferred choice. Researchers can avoid the complexities associated with correcting for temperature-induced volume changes in molarity.
Let’s summarize the real-life applications:
| Application | Molarity | Molality |
|---|---|---|
| Pharmaceutical Industry | Ensuring drug dosage accuracy | Used in specific cases, e.g., cryogenic research |
| Food and Beverage Industry | Measuring additives in products | |
| Environmental Monitoring | Analyzing water pollutants | |
| Cryogenics | Maintaining constant concentration at low temps | |
| Chemical Reactions | Ensuring consistent concentrations in reactions | Precision in temperature-sensitive reactions |
| Laboratory Research | General use | Preferred for precision and reproducibility |
4. Effects of Temperature Changes
Molarity:
As mentioned earlier, molarity is influenced by temperature changes because it is based on the volume of the solution. When the temperature rises, most substances expand, including liquids. This expansion leads to an increase in volume, which, in turn, affects the molarity.
To account for temperature-induced changes in molarity, chemists often perform temperature corrections. This involves measuring the solution’s volume at the reference temperature (usually 25°C or 298 K) and adjusting the molarity accordingly.
Let’s take a closer look at this by considering an example:
Example: You prepare a solution with a molarity of 1.0 M at 25°C. If you measure the same solution at 35°C without any temperature correction, the volume will appear larger, and the calculated molarity will be lower than 1.0 M. To obtain the accurate molarity at 35°C, you need to adjust for the temperature difference.
Molality:
Unlike molarity, molality is not affected by temperature variations. It relies solely on the mass of the solvent, which remains relatively constant with temperature changes.
This temperature independence is particularly advantageous in situations where precise measurements are critical, such as in chemical reactions and experiments conducted at extreme temperatures.
To illustrate this point, consider the same example as before:
Example: You prepare a solution with a molality of 1.0 mol/kg at 25°C. If you measure the same solution at 35°C, you won’t need to make any temperature corrections. The molality will remain constant at 1.0 mol/kg, regardless of the temperature change.
Molarity or Molality: Which One is Right Choose for You?
In the realm of chemistry, selecting the appropriate concentration measure can significantly impact the accuracy of your experiments and the success of your scientific endeavors. Molarity (M) and Molality (m) are two essential concepts, each with its unique strengths and applications. To determine which one is right for you, let’s explore the key factors to consider:
Molarity (M):
- Volume Matters: Molarity is all about volume. It measures the moles of solute per liter of solution. If your experiments involve precise control of solution volume or if you work in industries like pharmaceuticals or food and beverages, where volume-based measurements are practical, Molarity might be your ideal choice.
- Temperature Sensitivity: Keep in mind that Molarity is temperature-sensitive. Temperature fluctuations can affect the volume of your solution, leading to changes in concentration. If you can manage temperature corrections and need a straightforward concentration measure, Molarity is valuable.
Molality (m):
- Mass Takes Center Stage: Molality focuses on mass. It measures the moles of solute per kilogram of solvent. If you’re working with temperature-sensitive experiments, such as cryogenics or specific chemical reactions, where maintaining a constant concentration is crucial, Molality shines.
- Temperature Independence: Unlike Molarity, Molality remains constant with temperature changes. This feature is particularly advantageous in situations where precision is paramount.
To make an informed choice, assess your specific experimental needs. Consider whether your experiments require temperature stability, precise volume control, or if you’re working in industries with particular concentration requirements. In some cases, you might even find that using both Molarity and Molality, depending on the experiment’s nature, offers the best of both worlds. So, whether you’re crafting pharmaceutical formulas, exploring the mysteries of extreme temperatures, or conducting groundbreaking research, choosing between Molarity and Molality ultimately depends on your unique scientific journey.
FAQs
The fundamental difference lies in what they measure. Molarity (M) quantifies the moles of solute per liter of solution, while Molality (m) quantifies the moles of solute per kilogram of solvent.
Molarity is affected by temperature because it depends on the volume of the solution, which can change with temperature fluctuations. Adjustments are often required to correct for temperature-induced volume changes.
Molarity finds applications in various industries, including pharmaceuticals, food and beverages, and environmental analysis. It’s favored when precise control of chemical concentrations is needed.
Molality is preferred in temperature-sensitive cases, such as cryogenic applications and chemical reactions where maintaining a constant concentration despite temperature changes is crucial.
Yes, there are conversion formulas, but they require considering the density of the solution due to volume dependence when converting from Molarity to Molality.
Molarity is used in osmotic pressure calculations, while Molality is directly related to boiling-point elevation calculations, highlighting their distinct roles in colligative properties.
“M = 1.0 M” represents Molarity, indicating that there is 1 mole of solute per liter of solution. “m = 1.0 mol/kg” represents Molality, indicating that there is 1 mole of solute per kilogram of solvent.
Converting between the two may require accounting for the density of the solution when switching from Molarity to Molality. The conversion from Molality to Molarity is more straightforward, assuming relatively constant solution density.
Read More:
Contents






