| Characteristic | Conjugate Acids | Conjugate Bases |
|---|---|---|
| Definition | Substances formed by donating a proton (H+) in a chemical reaction. | Substances formed by accepting a proton (H+) in a chemical reaction. |
| Proton Exchange | Donate protons (H+). | Accept protons (H+). |
| Effect on pH | Increase the acidity of a solution. | Increase the basicity of a solution. |
| Reaction Direction | Tend to donate protons in a reverse reaction. | Tend to donate the proton they accepted in a reverse reaction. |
| Charge | Carry a positive charge. | Carry a negative charge. |
| Strength | Can be strong or weak acids, inversely related to the strength of their parent bases. | Can be strong or weak bases, generally related to the strength of their parent acids. |
| Stability and Resonance | Resonance stabilization may affect their stability. | Resonance stabilization may affect their stability. |
| Solubility and Precipitation | Can form insoluble salts in some cases. | Typically more soluble in water. |
| Lewis Acid-Base Behavior | Can act as Lewis acids by accepting electron pairs. | Can act as Lewis bases by donating electron pairs. |
| Equilibrium Constants (Ka, Kb) | Ka values indicate the strength of the acid. | Kb values indicate the strength of the base. |
| Biological Implications | Play roles in buffer systems and enzymatic reactions. | Essential for maintaining physiological pH and enzymatic reactions. |
| Environmental Impact | Relevant in water treatment processes and acid rain formation. | Involved in acid rain formation and water quality control. |
Imagine chemistry as a grand puzzle, and within it, we find Conjugate Acids and Conjugate Bases – two puzzle pieces that fit together in a mesmerizing dance of proton exchange. Conjugate Acids, akin to noble donors, offer up their protons (H+) during chemical reactions, while Conjugate Bases graciously accept these protons with open arms.
Differences Between Conjugate Acid and Conjugate Base
The main differences between Conjugate Acids and Conjugate Bases lie in their roles during chemical reactions. Conjugate Acids donate protons (H+ ions), whereas Conjugate Bases accept these protons. This fundamental distinction has profound effects: Conjugate Acids increase the acidity of a solution, while Conjugate Bases elevate its basicity. Additionally, the direction of their reactions and their resulting charges vary—Conjugate Acids tend to revert by donating the proton they acquired, carrying a positive charge, while Conjugate Bases tend to return the proton they accepted, bearing a negative charge. These disparities are at the core of acid-base chemistry and have wide-ranging implications in fields from biology to environmental science.
What Are Conjugate Acids and Conjugate Bases?
Before diving into the differences between conjugate acids and conjugate bases, let’s first grasp the basic concepts.
Conjugate Acids
Imagine you have a substance, let’s call it “Substance A,” which can donate a proton (H+) in a chemical reaction. When Substance A donates this proton, it transforms into a new substance, which we label as “Substance B.” This transformed Substance B is referred to as the conjugate acid. In essence, a conjugate acid is formed when a base (Substance A) gains a proton.
Conjugate Bases
Now, consider another scenario. You have a substance called “Substance C,” which has the ability to accept a proton (H+) during a chemical reaction. When Substance C receives this proton, it undergoes a transformation and becomes a new substance, which we’ll name “Substance D.” This transformed Substance D is known as the conjugate base. In simple terms, a conjugate base is created when an acid (Substance C) loses a proton.
To put it even more plainly, think of conjugate acids and conjugate bases as pairs that are linked by the exchange of a proton. When one substance loses a proton, it becomes its conjugate base, and when another substance gains a proton, it becomes its conjugate acid. Now that we’ve established the fundamental definitions, let’s delve into the key differences between these two essential components of acid-base chemistry.
1. Proton Donation vs. Proton Acceptance
The most fundamental difference between conjugate acids and conjugate bases lies in their role regarding protons (H+).
- Conjugate Acids: These substances are capable of donating protons to other molecules. In other words, they have an extra proton that they can part with during a chemical reaction. This donation of protons makes them more positively charged.
- Conjugate Bases: On the flip side, conjugate bases are skilled at accepting protons from other molecules. They have the capacity to accommodate an additional proton, which means they tend to be negatively charged after accepting a proton.
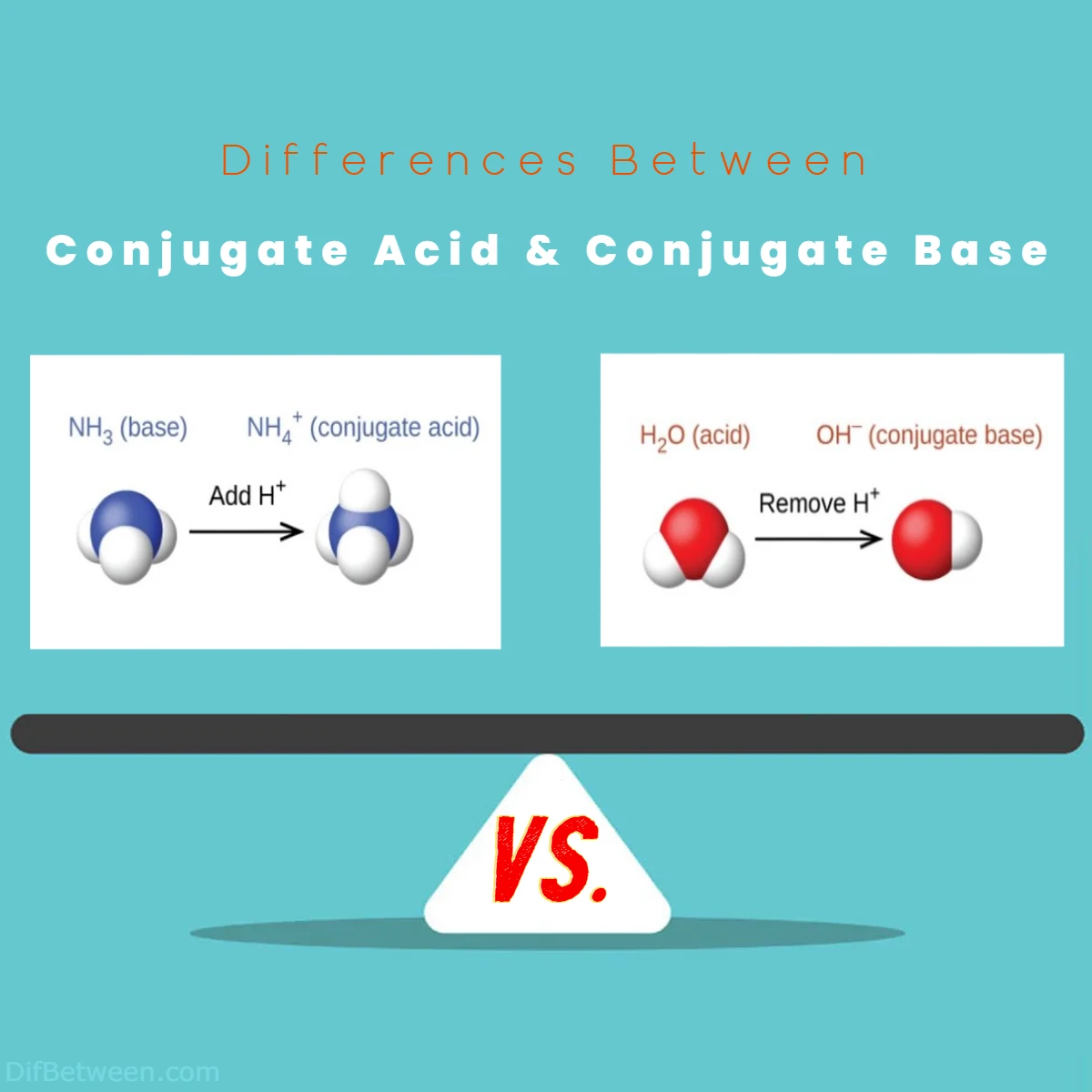
To illustrate this difference, let’s take a look at a classic example involving water (H2O) and its conjugate acid-base pair:
- Water (H2O): In this scenario, water acts as a base by accepting a proton (H+) from another molecule. When this happens, water transforms into its conjugate acid, which is known as the hydronium ion (H3O+). The reaction can be represented as follows:H2O + H+ → H3O+
- Hydronium Ion (H3O+): This newly formed species is the conjugate acid because it has gained an extra proton (H+). It’s more positively charged than the original water molecule.
So, in summary, water (H2O) is the base, and the hydronium ion (H3O+) is its conjugate acid.
2. pH Level Influence
The presence of conjugate acids and conjugate bases has a direct impact on the pH of a solution. pH is a measure of how acidic or basic a substance is, and it’s determined by the concentration of hydrogen ions (H+) in a solution.
- Conjugate Acids: When a conjugate acid is added to a solution, it increases the concentration of hydrogen ions (H+), making the solution more acidic. This is because conjugate acids readily donate protons, leading to a higher H+ concentration.
- Conjugate Bases: Conversely, when a conjugate base is introduced into a solution, it decreases the concentration of hydrogen ions (H+), making the solution more basic. Conjugate bases do this by accepting protons, which reduces the H+ concentration.
To provide a practical example, consider the behavior of the bicarbonate ion (HCO3-) and its conjugate acid, carbonic acid (H2CO3), in the context of blood chemistry:
- Bicarbonate Ion (HCO3-): In our blood, the bicarbonate ion acts as a conjugate base. It helps regulate blood pH by accepting protons (H+) in the bloodstream. This helps to keep our blood slightly basic, which is crucial for normal physiological functions.
- Carbonic Acid (H2CO3): Carbonic acid is the conjugate acid of the bicarbonate ion. It donates protons (H+) when needed to maintain the delicate pH balance in the blood. When the blood becomes too basic, carbonic acid donates protons to bring the pH back to a healthy range.
3. Reaction Direction
Another key difference between conjugate acids and conjugate bases is the direction in which chemical reactions tend to proceed.
- Conjugate Acids: When a substance acts as a conjugate acid in a reaction, it implies that it has accepted a proton and is now ready to donate it back in the reverse reaction. In other words, conjugate acids have a tendency to revert to their original form by donating the acquired proton.
- Conjugate Bases: Conversely, conjugate bases have accepted a proton and are now poised to give it away in a reverse reaction. They tend to return to their initial state by donating the proton they received.
This dynamic equilibrium between conjugate acids and bases is beautifully exemplified in the behavior of the ammonium ion (NH4+) and ammonia (NH3):
- Ammonium Ion (NH4+): In a reaction, ammonia (NH3) can act as a base by accepting a proton (H+) to become the ammonium ion (NH4+).
- Ammonia (NH3): This is the conjugate base of the ammonium ion. It can donate the proton it received, reverting back to the ammonium ion.
The equilibrium between NH3 and NH4+ is represented as follows:
NH3 + H+ ⇌ NH4+
4. Charge
The charges associated with conjugate acids and conjugate bases are worth noting.
- Conjugate Acids: These species tend to carry a positive charge because they have gained a proton (H+), which contributes an additional positive charge. Therefore, conjugate acids are cations (positively charged ions).
- Conjugate Bases: In contrast, conjugate bases typically bear a negative charge due to their acceptance of a proton (H+), which adds an extra negative charge. Consequently, conjugate bases are anions (negatively charged ions).
To demonstrate this concept, let’s look at the chloride ion (Cl-) and its conjugate acid, hydrochloric acid (HCl):
- Chloride Ion (Cl-): In this case, Cl- acts as the conjugate base. It has gained a proton (H+) to become the chloride ion, which carries a negative charge.
- Hydrochloric Acid (HCl): Hydrochloric acid is the conjugate acid of the chloride ion. It can donate the proton it acquired, reverting back to chloride ion.
The transformation between Cl- and HCl can be summarized as:
Cl- + H+ ⇌ HCl
5. Strength and Weakness
Conjugate acids and conjugate bases can vary in their strength, which relates to their ability to donate or accept protons.
- Conjugate Acids: Some conjugate acids are strong, meaning they readily donate protons in solution. Others are weak and don’t donate protons as easily. The strength of a conjugate acid is often inversely related to the strength of its parent base.
- Conjugate Bases: Similar to conjugate acids, some conjugate bases are strong and readily accept protons, while others are weak and don’t accept protons as easily. The strength of a conjugate base is generally related to the strength of its parent acid.
Table 1 below provides examples of strong and weak conjugate acid-base pairs:
Table 1: Examples of Strong and Weak Conjugate Acid-Base Pairs
| Conjugate Acid | Conjugate Base | Strength |
|---|---|---|
| Hydrochloric Acid (HCl) | Chloride Ion (Cl-) | Strong |
| Sulfuric Acid (H2SO4) | Hydrogen Sulfate Ion (HSO4-) | Weak |
| Ammonium Ion (NH4+) | Ammonia (NH3) | Strong |
| Acetic Acid (CH3COOH) | Acetate Ion (CH3COO-) | Weak |
In summary, the strength of conjugate acids and bases is an important consideration in acid-base chemistry, as it impacts the overall reactivity of these species.
6. Examples in Everyday Life
To better grasp the concept of conjugate acids and conjugate bases, it’s helpful to explore some common examples from our daily lives.
Conjugate Acids in Household Items
- Vinegar (Acetic Acid): Vinegar is a common household item that contains acetic acid (CH3COOH). When acetic acid donates a proton, it becomes its conjugate base, the acetate ion (CH3COO-).
- Lemon Juice (Citric Acid): Citric acid is found in lemon juice and other citrus fruits. When citric acid donates a proton, it forms the citrate ion (C6H5O7-), which is its conjugate base.
Conjugate Bases in Biological Systems
- Blood Buffer System: As mentioned earlier, the bicarbonate ion (HCO3-) acts as a conjugate base in our blood’s buffer system. It helps maintain the blood’s pH within a narrow and healthy range.
- Amino Acids: Amino acids, the building blocks of proteins, contain both acidic and basic functional groups. When they lose or gain protons, they can act as either conjugate acids or conjugate bases, depending on the conditions in their surroundings.
These real-world examples demonstrate how conjugate acids and bases play crucial roles in various aspects of our lives, from food preparation to the functioning of our bodies.
7. Stability and Resonance
The stability of conjugate acids and conjugate bases can vary significantly and is influenced by factors like resonance and electron delocalization.
- Conjugate Acids: In some cases, conjugate acids can be more stable due to resonance effects. Resonance occurs when electrons are delocalized or shared among multiple atoms within a molecule, creating multiple resonance structures. When a conjugate acid has resonance stabilization, it is less likely to release its proton, making it a weaker acid.Example: The carboxylic acid group, found in compounds like acetic acid (CH3COOH), has resonance stabilization. This makes acetic acid a weaker acid than non-resonance-stabilized acids, like hydrochloric acid (HCl).
- Conjugate Bases: Conversely, conjugate bases with resonance stabilization tend to be more stable and less reactive. This stability makes them better at accepting protons, making them stronger bases.Example: The acetate ion (CH3COO-) is a conjugate base with resonance stabilization. It is more stable than non-resonance-stabilized bases, such as hydroxide ion (OH-).
8. Solubility and Precipitation
The solubility of compounds containing conjugate acids and conjugate bases can vary, leading to differences in their behavior in solution.
- Conjugate Acids: Some conjugate acids can form insoluble salts when they react with certain anions. This can result in the precipitation of the salt from the solution. The solubility of a conjugate acid salt depends on the specific ions involved.Example: If you mix a solution of silver nitrate (AgNO3) with hydrochloric acid (HCl), it forms the insoluble silver chloride (AgCl) precipitate due to the reaction:Ag+ (from AgNO3) + Cl- (from HCl) → AgCl (precipitate)
- Conjugate Bases: Conjugate bases, on the other hand, are typically more soluble in water than their corresponding conjugate acids. This is because many conjugate bases are negatively charged ions, which can be stabilized by the polar water molecules.Example: The chloride ion (Cl-) is the conjugate base of hydrochloric acid (HCl) and is highly soluble in water.
9. Lewis Acids and Bases
In addition to their roles as Bronsted-Lowry acids and bases (which involve proton transfer), conjugate acids and conjugate bases also have relevance in Lewis acid-base reactions.
- Conjugate Acids as Lewis Acids: In Lewis acid-base theory, a Lewis acid is a species that can accept an electron pair. Conjugate acids can act as Lewis acids because they have vacant orbitals or electron-deficient sites that can accept electron pairs from Lewis bases.Example: Boron trifluoride (BF3) is a Lewis acid that can accept an electron pair from a Lewis base, such as ammonia (NH3), to form a Lewis acid-base adduct.
- Conjugate Bases as Lewis Bases: Conversely, conjugate bases can function as Lewis bases because they have lone pairs of electrons that can be donated to Lewis acids.Example: The hydroxide ion (OH-) can act as a Lewis base by donating its lone pair of electrons to a Lewis acid, such as a proton (H+), to form water (H2O).
10. Chemical Reactions and Equilibrium Constants
In chemical reactions involving acids and bases, the equilibrium constant (K) can provide insights into the relative strengths of the reactants and products. Understanding the K values for conjugate acid-base pairs can be instructive.
- Conjugate Acids and Equilibrium Constants: When an acid (HA) donates a proton to water (H2O), it forms its conjugate base (A-) and hydronium ion (H3O+). The equilibrium constant (Ka) for this reaction is an indicator of the strength of the acid. A larger Ka value indicates a stronger acid.Example: The equilibrium constant for acetic acid (CH3COOH) donating a proton to water is represented as follows:CH3COOH + H2O ⇌ CH3COO- + H3O+The larger the Ka value, the stronger the acetic acid as a donor of protons.
- Conjugate Bases and Equilibrium Constants: The equilibrium constant (Kb) for the reaction of a base (B) with water to form its conjugate acid (BH+) and hydroxide ion (OH-) can indicate the strength of the base. A larger Kb value signifies a stronger base.Example: The equilibrium constant for ammonia (NH3) accepting a proton from water is represented as follows:NH3 + H2O ⇌ NH4+ + OH-A higher Kb value indicates that ammonia is a stronger base.
11. Biological Implications
Conjugate acids and bases play crucial roles in various biological processes. Understanding these roles is vital for comprehending biochemical reactions and maintaining physiological homeostasis.
- Buffer Systems: Biological systems often rely on buffer systems to maintain a stable pH. These systems involve conjugate acid-base pairs that can accept or donate protons to prevent drastic changes in pH.Example: In the human body, the bicarbonate buffer system involves the interconversion of carbonic acid (H2CO3) and bicarbonate ions (HCO3-) to help regulate blood pH.
- Enzymatic Reactions: Many enzymatic reactions in biological organisms involve the participation of conjugate acids and bases. Enzymes often act as catalysts by donating or accepting protons to facilitate chemical transformations.Example: Enzymes called proteases catalyze the hydrolysis of peptide bonds in proteins. In these reactions, water (H2O) can act as a conjugate base, accepting a proton from the peptide bond.
12. Environmental Impact
Conjugate acids and bases can also have environmental implications, particularly in the context of water quality and pollution.
- Water Treatment: Understanding the behavior of conjugate acids and bases is essential in water treatment processes. Adjusting the pH of water is often necessary to remove impurities and ensure safe drinking water.Example: In water treatment plants, chemicals like sodium hydroxide (NaOH) are added to raise the pH and precipitate impurities as solids.
- Acid Rain: Acid rain is a form of environmental pollution that results from the release of acidic pollutants into the atmosphere. These pollutants can react with water to form conjugate acids, which can have detrimental effects on ecosystems and infrastructure.Example: Sulfur dioxide (SO2) released from burning fossil fuels can react with atmospheric water to form sulfuric acid (H2SO4), contributing to acid rain.
Conclusion
In this expanded exploration of conjugate acids and conjugate bases, we’ve uncovered a myriad of facets that distinguish these chemical entities. From their stability and resonance effects to their roles in Lewis acid-base reactions, chemical equilibria, biological processes, and environmental impact, conjugate acids and bases are central to our understanding of chemistry and its practical applications.
Whether you’re a student embarking on a chemistry course, a researcher investigating biochemical pathways, or simply someone curious about the world of acids and bases, the knowledge of conjugate acids and bases will continue to enrich your understanding of the chemical and biological processes that shape our world.
FAQs
A conjugate acid is a chemical species that forms when a base accepts a proton (H+) during a chemical reaction. It is derived from its parent base by gaining a proton.
A conjugate base is a chemical species that forms when an acid donates a proton (H+) during a chemical reaction. It is derived from its parent acid by losing a proton.
Conjugate acids tend to increase the acidity of a solution when added, while conjugate bases tend to increase the basicity of a solution. Their influence on pH is significant in acid-base chemistry.
Yes, both conjugate acids and conjugate bases can be either strong or weak, depending on their ability to donate or accept protons. The strength of a conjugate acid is often inversely related to the strength of its parent base, and vice versa.
Common examples include water (H2O) and the hydronium ion (H3O+), where water is the base and the hydronium ion is its conjugate acid. Another example is ammonia (NH3) and the ammonium ion (NH4+), with ammonia as the base and the ammonium ion as its conjugate acid.
Conjugate acids and bases play vital roles in maintaining pH balance in biological systems, such as blood buffering, enzymatic reactions, and cellular processes, ensuring the proper functioning of living organisms.
Yes, conjugate acids and bases have environmental significance, particularly in processes like water treatment and the formation of acid rain. Understanding their behavior is essential for addressing pollution and maintaining water quality.
Ka (acid dissociation constant) is associated with the strength of an acid, while Kb (base dissociation constant) indicates the strength of a base. These constants are used to quantify the extent of ionization of acids and bases in solution.
Yes, some conjugate acids and bases can exhibit resonance stabilization, which can affect their stability and reactivity. Resonance occurs when electrons are delocalized within a molecule, leading to multiple resonance structures.
To gain a comprehensive understanding of these differences and their significance in various fields, continue exploring our detailed guide, “Differences Between Conjugate Acid vs Conjugate Base.”
Read More:
Contents
- Differences Between Conjugate Acid and Conjugate Base
- What Are Conjugate Acids and Conjugate Bases?
- 1. Proton Donation vs. Proton Acceptance
- 2. pH Level Influence
- 3. Reaction Direction
- 4. Charge
- 5. Strength and Weakness
- 6. Examples in Everyday Life
- 7. Stability and Resonance
- 8. Solubility and Precipitation
- 9. Lewis Acids and Bases
- 10. Chemical Reactions and Equilibrium Constants
- 11. Biological Implications
- 12. Environmental Impact
- Conclusion
- FAQs






