| Property | Fluorine | Fluoride Compounds |
|---|---|---|
| Chemical Identity | Element (F) | Chemical Compounds (e.g., NaF, CaF₂) |
| Nature | Reactive gas (F₂) | Ionic compounds (F⁻ combined with other elements) |
| Reactivity | Highly reactive with most elements | Generally less reactive than fluorine |
| State at Room Temperature | Gas | Solid, liquid, or gas (depending on the compound) |
| Toxicity | Extremely toxic when inhaled | Generally less toxic than pure fluorine |
| Color and Odor | Pale yellow-green, pungent odor | Varies depending on the compound |
| Natural Occurrence | Rare in nature | Naturally occurring in minerals and water sources |
| Applications | Limited due to extreme reactivity | Wide range of industrial and dental applications |
| Dental Health | Not used for dental health | Essential for preventing tooth decay |
| Environmental Impact | Air and water pollution, soil contamination | Environmental monitoring, regulated for safety |
Did you know that fluorine is a gas so reactive that it can make glass cringe? Or that fluoride, its tamer counterpart, is your oral health ally, diligently guarding your teeth against the relentless onslaught of cavities? Yes, these elements and compounds have quite the story to tell, and we’re here to decipher their tales.
Differences Between Fluorine and Fluoride
The main differences between Fluorine vs Fluoride lie in their nature and reactivity. Fluorine is a highly reactive chemical element, while Fluoride is a chemical compound that contains the fluorine ion (F⁻). Fluorine exists as a diatomic gas, is toxic, and readily reacts with almost all other elements. In contrast, fluoride compounds are generally less reactive and can exist in various states, such as solids, liquids, or gases, depending on their composition. Fluoride is commonly used in dental care, water treatment, and various industries, while fluorine’s extreme reactivity limits its direct applications, making it essential primarily through its compounds.
Understanding the Basics
Fluorine: The Reactive Element
Fluorine, with the chemical symbol F and atomic number 9, is a highly reactive chemical element. It is the lightest halogen and exists as a diatomic gas under standard conditions. Fluorine is a member of the halogen group in the periodic table, which also includes chlorine, bromine, iodine, and astatine.
Properties of Fluorine:
- Reactivity: Fluorine is renowned for its extreme reactivity. It readily forms compounds with almost all other elements, earning its reputation as one of the most reactive elements in the periodic table.
- Toxicity: Pure fluorine gas is highly toxic and can be lethal if inhaled. It’s so reactive that it can even react with substances as benign as glass.
- Color and Odor: Fluorine gas is pale yellow-green in color and has a pungent, acrid odor.
- Applications: Due to its extreme reactivity, elemental fluorine is not typically used in its pure form. Instead, its compounds, such as fluorides, are essential in various industries, including dentistry, metallurgy, and electronics.
Fluoride: The Chemical Compound
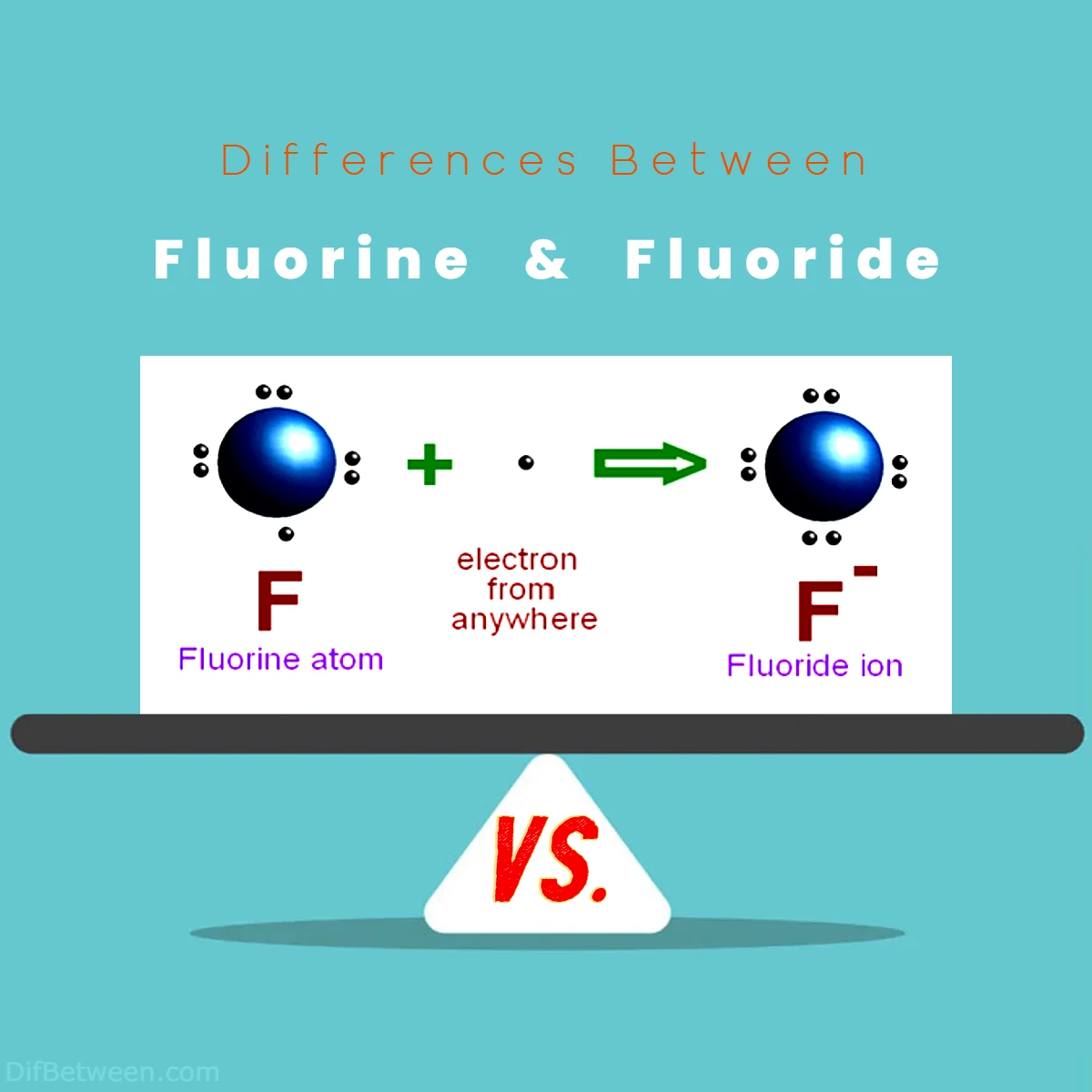
Fluoride, on the other hand, is not an element but a chemical compound that contains the fluorine ion (F⁻). It is formed when fluorine, the reactive element, reacts with other elements to create compounds. These compounds are collectively referred to as fluorides.
Properties of Fluoride:
- Ionic Nature: Fluoride is an anion, meaning it carries a negative charge (F⁻). It readily forms ionic bonds with positively charged ions (cations) to create stable compounds.
- Stability: Unlike its parent element fluorine, fluoride compounds are often stable and have a wide range of applications.
- Common Sources: Fluoride is naturally present in various minerals and water sources. It is also commonly added to toothpaste and drinking water for dental health purposes.
- Applications: Fluoride compounds are used in many industries, including water treatment, metallurgy, and the production of ceramics and glass.
The Key Differences
Now that we’ve covered the basics, let’s delve deeper into the key differences between fluorine and fluoride.
1. Nature
Fluorine: Fluorine is a chemical element, which means it consists of only one type of atom – fluorine atoms. It is found in nature as a diatomic molecule, F₂, and exists as a gas under standard conditions.
Fluoride: Fluoride, on the other hand, is not an element but a chemical compound. It is composed of the fluorine ion (F⁻) combined with one or more other elements. Fluoride compounds can be solids, liquids, or gases, depending on their composition.
2. Reactivity
Fluorine: As mentioned earlier, fluorine is one of the most reactive elements in the periodic table. It readily reacts with almost all other elements, and its reactivity is so high that it can even react with glass. This extreme reactivity makes elemental fluorine challenging to handle safely.
Fluoride: Fluoride compounds, in contrast, are generally much less reactive than elemental fluorine. While they can still participate in chemical reactions, they are not as dangerously reactive as pure fluorine gas. This reduced reactivity makes fluoride compounds more practical for various applications.
3. State at Room Temperature
Fluorine: Fluorine exists as a diatomic gas (F₂) at room temperature and standard pressure (25°C and 1 atmosphere). It does not have a liquid or solid form under these conditions.
Fluoride: Fluoride compounds can exist in various states at room temperature, depending on their specific chemical composition. Some fluoride compounds are solids, such as sodium fluoride (NaF), while others may be liquids or gases under similar conditions.
4. Toxicity
Fluorine: Pure fluorine gas is highly toxic and poses a significant health hazard if inhaled. Handling elemental fluorine requires extreme caution and specialized equipment due to its toxicity and reactivity.
Fluoride: Fluoride compounds are generally not as toxic as pure fluorine gas. In fact, fluoride ions are used in dentistry to promote dental health. However, like many chemical compounds, excessive ingestion of certain fluoride compounds can lead to health issues, such as dental fluorosis or skeletal fluorosis.
5. Uses and Applications
Fluorine: Elemental fluorine is not used directly in most industrial or commercial applications due to its extreme reactivity and toxicity. Instead, it is used indirectly through its compounds, particularly fluorides.
Fluoride: Fluoride compounds have a wide range of applications across various industries:
- Dental Care: Fluoride is added to toothpaste and drinking water to prevent tooth decay and promote oral health.
- Metallurgy: Fluorides are used in the extraction and refining of metals, such as aluminum and uranium.
- Water Treatment: Fluoride is added to drinking water in controlled amounts to improve dental health.
- Chemical Industry: Fluoride compounds are essential in the production of various chemicals, including hydrofluoric acid and fluorocarbon compounds used in refrigeration and air conditioning.
- Ceramics and Glass: Fluoride compounds enhance the properties of ceramics and glass, making them more durable and heat-resistant.
6. Natural Occurrence
Fluorine: Elemental fluorine is extremely rare in nature due to its high reactivity. It is primarily found as a component of certain minerals, such as fluorite (calcium fluoride), but it does not exist in significant quantities on its own.
Fluoride: Fluoride ions (F⁻) are naturally occurring and can be found in various minerals, soils, and water sources. Some regions have naturally fluoridated groundwater, which can be a source of fluoride for drinking water.
Comparing Physical and Chemical Properties
To further understand the disparities between fluorine and fluoride, let’s compare their physical and chemical properties in a tabular format:
| Property | Fluorine | Fluoride Compounds |
|---|---|---|
| Chemical Symbol | F | Varies |
| Nature | Element | Chemical Compounds |
| Reactivity | Highly Reactive | Variable (Less Reactive) |
| State at Room Temp. | Gas (F₂) | Solid, Liquid, or Gas (Varies) |
| Toxicity | Extremely Toxic | Varies (Generally Less Toxic) |
| Uses and Applications | Limited (Through Compounds) | Diverse (Water treatment, Metallurgy, Dental care, etc.) |
| Natural Occurrence | Rare | Common (in minerals, water sources) |
As shown in the table above, fluorine and fluoride differ significantly in their properties and applications.
Safety and Precautions
Now that we’ve explored the key differences between fluorine and fluoride, it’s crucial to emphasize the importance of safety and precautions when dealing with these substances.
Safety with Fluorine
Handling elemental fluorine is a task that demands extreme caution and specialized equipment due to its toxicity and reactivity. Here are some essential safety precautions when working with fluorine:
- Proper Ventilation: Work in a well-ventilated area to prevent the accumulation of fluorine gas, which can be hazardous.
- Use of Protective Gear: Wear appropriate personal protective equipment (PPE), including chemical-resistant gloves, goggles, and a lab coat.
- Specialized Equipment: Employ specialized equipment made of materials that resist fluorine’s corrosive effects, such as Teflon or stainless steel.
- Avoid Contamination: Prevent contamination of equipment and surfaces. Even trace amounts of reactive substances can lead to unexpected reactions.
- Gas Monitoring: Continuously monitor fluorine gas levels in the workspace to ensure safe concentrations.
- Emergency Response: Have a well-defined emergency response plan in case of accidents or leaks, including access to emergency eyewash stations and showers.
Safety with Fluoride Compounds
Fluoride compounds are generally less toxic and reactive than pure fluorine, but they still require care when handling. Here are some safety precautions for working with fluoride compounds:
- Avoid Ingestion: Do not ingest fluoride compounds. Ingestion of large amounts can lead to health issues, including fluoride poisoning.
- Proper Storage: Store fluoride compounds in well-labeled containers in a cool, dry place away from incompatible substances.
- Use PPE: Depending on the specific compound and application, wear appropriate personal protective equipment, including gloves and goggles.
- Dust Control: Minimize the generation of dust when working with powdered fluoride compounds to prevent inhalation.
- First Aid: Know the appropriate first-aid measures in case of contact with fluoride compounds, such as rinsing with water or seeking medical attention.
- Follow Guidelines: Adhere to safety guidelines and recommendations provided by regulatory agencies and your organization.
Environmental Impact
Understanding the environmental impact of fluorine and fluoride is crucial, especially given their widespread use in various industries. Here’s an overview of their environmental considerations:
Environmental Impact of Fluorine
- Air Pollution: Fluorine gas is released into the atmosphere during certain industrial processes, contributing to air pollution. It can react with other compounds, forming secondary pollutants.
- Water Pollution: Accidental releases of fluorine can contaminate water bodies. Fluorine compounds in water can be toxic to aquatic life.
- Soil Contamination: When fluorine-containing compounds are used in agriculture or as industrial additives, they can accumulate in soil, potentially affecting plant growth and soil quality.
- Long-term Effects: The long-term environmental impact of fluorine emissions can lead to ecosystem disturbances, especially in regions with heavy industrial activity.
Environmental Impact of Fluoride Compounds
- Water Fluoridation: The addition of fluoride to drinking water, a common practice to improve dental health, is carefully regulated to maintain safe levels. However, excessive fluoride in drinking water can lead to adverse health effects and fluorosis in humans.
- Soil and Agriculture: The use of fluoride-containing fertilizers or pesticides can introduce fluoride into soil. Proper management is necessary to prevent soil contamination and its potential effects on crops.
- Ecosystem Effects: High levels of fluoride in the environment can negatively impact aquatic ecosystems, particularly sensitive species like amphibians.
- Waste Disposal: Proper disposal of fluoride-containing waste materials is essential to prevent groundwater contamination and ecosystem disruption.
The Future of Fluorine and Fluoride
As science and technology continue to advance, so do our understanding and applications of fluorine and fluoride. Here’s a glimpse into the future of these substances:
Research and Innovation
- Green Chemistry: Ongoing research aims to develop greener and more sustainable methods for working with fluorine and fluoride, reducing their environmental impact.
- Materials Science: Fluorine-based materials are being explored for their unique properties, such as superhydrophobic coatings and high-temperature superconductors.
- Drug Development: Fluorinated compounds are increasingly utilized in pharmaceuticals due to their potential for improved drug properties, such as increased stability and bioavailability.
Dental Health
- Precision Dentistry: Advances in dentistry may lead to more targeted and personalized approaches to using fluoride for preventing dental cavities.
- Fluoride Alternatives: Research continues into alternative dental treatments and materials that can achieve similar benefits to fluoride without potential health risks.
Environmental Regulation
- Stricter Regulations: Environmental agencies are likely to continue monitoring and regulating the levels of fluoride in water and industrial emissions to protect human health and ecosystems.
- Waste Management: Improved waste management practices for fluoride-containing waste materials will become increasingly important to mitigate environmental impacts.
FAQs
Fluorine is a chemical element with the symbol F and atomic number 9. It is a highly reactive diatomic gas and is considered one of the most reactive elements in the periodic table. Fluoride, on the other hand, is not an element but a chemical compound that contains the fluoride ion (F⁻). The key difference is that fluorine is a single element, whereas fluoride is a compound formed when fluorine reacts with other elements.
Yes, both fluorine and fluoride can be toxic, but their toxicity levels differ significantly. Fluorine gas is highly toxic and can be lethal if inhaled, making it extremely dangerous to handle. In contrast, fluoride compounds are generally less toxic, and their toxicity depends on the specific compound and its concentration. While excessive ingestion of certain fluoride compounds can lead to health issues, they are not as immediately dangerous as pure fluorine gas.
Fluoride plays a crucial role in dental care. It is added to toothpaste and often to drinking water in controlled amounts to help prevent tooth decay and promote oral health. Fluoride ions in dental products strengthen tooth enamel, making it more resistant to acid attacks from bacteria. Fluorine, as an extremely reactive element, is not used directly in dental care but indirectly through its compounds like fluoride.
Fluorine emissions from industrial processes can contribute to air pollution and water contamination if not properly managed. Fluoride compounds, when present in high concentrations in the environment, can negatively impact aquatic ecosystems and soil quality. Balancing the benefits of fluoride for dental health with its potential environmental impact is a subject of ongoing research and regulation.
Fluorine is rarely found in nature in its elemental form due to its high reactivity. It is more commonly found in the form of fluoride compounds in minerals and water sources. Some regions have naturally fluoridated groundwater, which can be a source of fluoride.
Fluoride compounds have diverse applications in various industries, including water treatment, metallurgy, and the production of ceramics and glass. They are also essential for dental health in toothpaste and drinking water. Fluorine, due to its extreme reactivity and toxicity, is not used directly in most applications but indirectly through its compounds, particularly fluorides.
Read More:
Contents