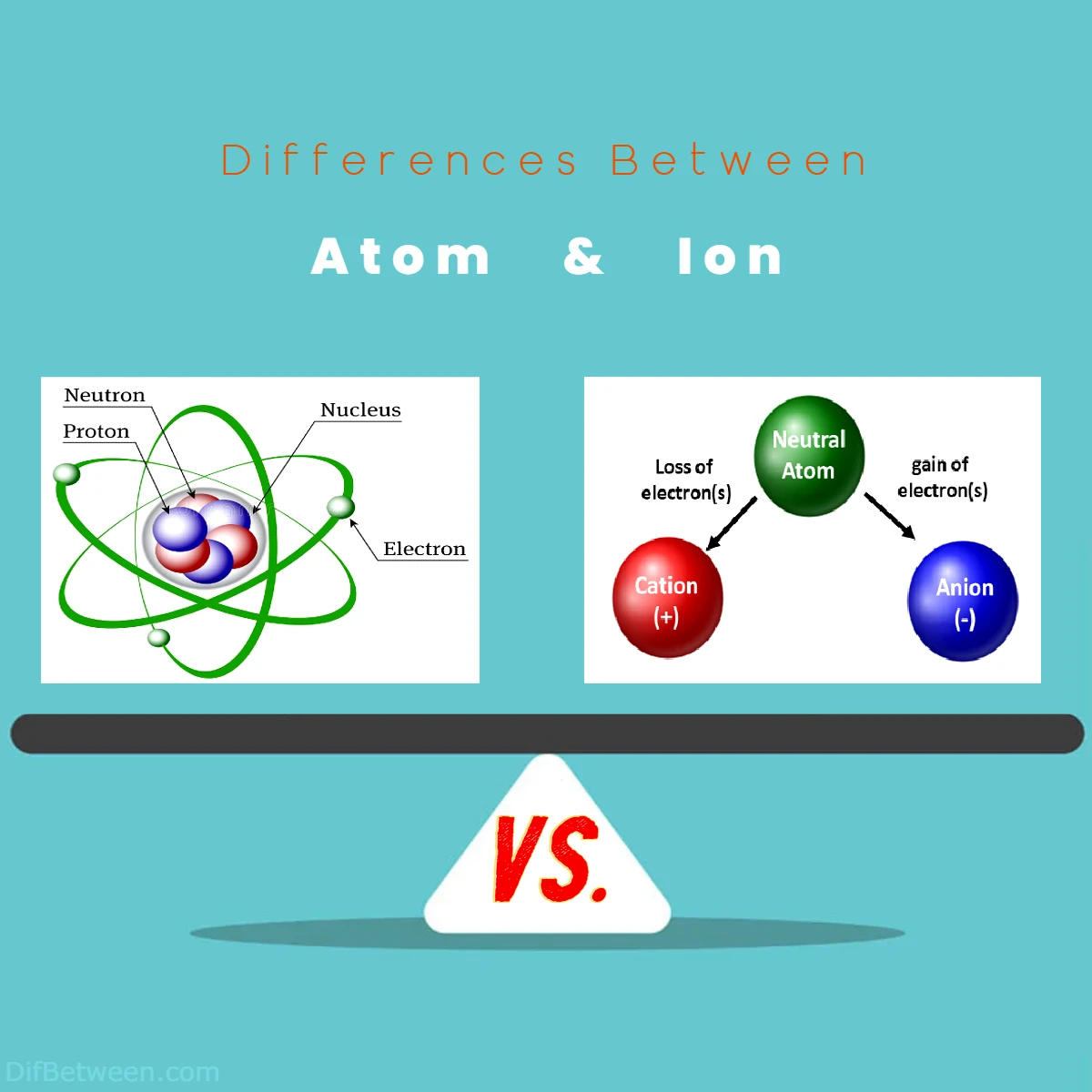
| Aspect | Atom | Ion |
|---|---|---|
| Definition | The fundamental unit of matter. | An atom or molecule with unequal protons and electrons. |
| Charge | Neutral (equal number of protons and electrons). | Positively (cation) or negatively (anion) charged due to lost or gained electrons. |
| Atomic Structure | Nucleus with protons and neutrons; electrons orbit around nucleus. | Nucleus with protons and neutrons; electrons orbit around nucleus, but arrangement changes due to charge. |
| Formation | Doesn’t change its electrons under normal conditions. | Gains or loses electrons to become charged and stable. |
| Behavior in Reactions | Determines chemical properties and reactivity based on electron configuration. | Bonds form due to attraction between opposite charges; behavior influenced by charge. |
| Size | Predictable size based on number of protons and electron distribution. | Cations are smaller (lost electrons), anions are larger (gained electrons). |
| Properties | Stable, electrically neutral, predictable size. | Charged, different size due to charge, conductive in solution. |
| Quantum Mechanics | Electrons have specific energy levels and orbitals. | Electron behavior changes due to charge; interactions between ions follow quantum principles. |
| Types | Different elements with varying properties. | Cations (positively charged) and anions (negatively charged); monoatomic and polyatomic ions. |
| Applications | Materials engineering, atomic spectroscopy. | Batteries, energy production, semiconductor manufacturing. |
| Forces | Electromagnetic forces in chemical interactions. | Electromagnetic forces, strong nuclear force in nucleus. |
| Isotopes | Same element, different atomic masses due to varying neutrons. | Not applicable. |
| Isotonicity | Not applicable. | Pertains to solutions with equal osmotic pressure. |
As we traverse the intricacies of atoms and ions, we’ll journey through their fundamental definitions, explore the dynamic interplay of charges, dive into their atomic structures, and witness the magic of quantum mechanics that governs their behavior. We’ll uncover the marvelous transformations that turn atoms into ions, creating entities with newfound electric personalities.
Differences Between Atom and Ion
The main differences between atoms and ions lie in their charges and structures. Atoms are the basic units of matter, consisting of a nucleus with protons and neutrons, orbited by electrons. They are electrically neutral, with equal protons and electrons. In contrast, ions are atoms or molecules with unequal protons and electrons, resulting in a positive (cation) or negative (anion) charge. Ions form through the gain or loss of electrons, influencing their behavior and properties. While atoms maintain their neutral state, ions exhibit charged interactions that play a crucial role in chemical reactions and the formation of compounds.
Atomic Structure: The Heart of the Matter
To understand the differences between atoms and ions, we need to dive into their atomic structures.
Atoms
Atoms consist of a nucleus at their core, where protons and neutrons reside, surrounded by a cloud of electrons in various energy levels or orbitals. Each element is defined by the number of protons in its nucleus, known as the atomic number. This number distinguishes one element from another on the periodic table.
The number of protons also determines the number of electrons in a neutral atom. For instance, a carbon atom with six protons will also have six electrons in its normal state, maintaining a balance of positive and negative charges.
Ions
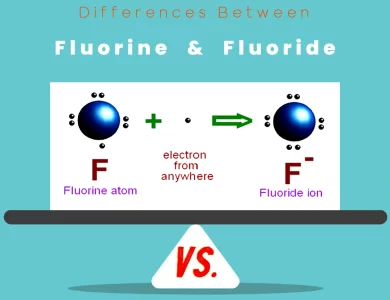
Ions, as mentioned earlier, are atoms that have gained or lost electrons. This process alters their charge and leads to unique properties. When an atom loses electrons, it becomes positively charged. Conversely, when it gains electrons, it becomes negatively charged.
Let’s take the example of sodium (Na) and chlorine (Cl). A sodium atom has 11 electrons, with the outermost electron in the third energy level. Chlorine, on the other hand, has 17 electrons, with the outermost electron in the third energy level as well. Sodium has a tendency to lose one electron, and chlorine has a tendency to gain one. When sodium loses an electron, it becomes a sodium cation (Na+), with 11 protons and 10 electrons. When chlorine gains an electron, it becomes a chloride anion (Cl-), with 17 protons and 18 electrons.
Charged Affairs: Ion Formation
The process of ion formation is a captivating tale of gaining and losing, resulting in charged entities that interact uniquely with their surroundings.
Atom to Ion Conversion
Atoms become ions through the gain or loss of electrons. This transformation occurs due to various factors, including chemical reactions and energy influences. An atom’s desire to attain stability drives this conversion. Remember, atoms are most stable when their outermost energy levels are either filled or empty.
Atoms with a few electrons in their outermost energy level tend to lose them to achieve stability. This is often seen in metals like sodium, which readily loses an electron to form a positively charged cation. On the other hand, atoms with almost full outer energy levels may gain electrons. For instance, oxygen, with six electrons in its outer level, yearns for two more electrons to complete its octet. By gaining two electrons, oxygen forms a negatively charged anion.
Ionic Bonds: The Force of Attraction
Ions, with their newfound charges, aren’t content to wander alone. They seek companionship through ionic bonds, an electromagnetic attraction between oppositely charged ions. These bonds are prevalent in compounds made up of elements with significantly different electronegativities, leading to the transfer of electrons from one atom to another.
Sodium chloride (NaCl), commonly known as table salt, is a classic example of ionic bonding. Sodium, with its positive charge, is irresistibly drawn to the negatively charged chloride. The result is a crystal lattice where countless sodium and chloride ions are held together by this strong ionic attraction.
Properties and Behavior: A Tale of Two Behaviors
The differences between atoms and ions shine through in their distinct properties and behaviors.
Atomic Properties
Atoms, in their natural state, are electrically neutral, with an equal number of protons and electrons. They have a predictable size, which is often measured in picometers (10^-12 meters). Atomic size varies as you move across or down the periodic table due to changes in electron distribution and energy levels.
The behavior of atoms in chemical reactions is determined by their electronic configuration. Atoms tend to form bonds to achieve a stable electron configuration, often following the octet rule, which states that atoms seek to have eight electrons in their outermost energy level.
Ionic Properties
Ions, with their charge, bring a new set of properties to the table. They’re not confined by the neutrality of atoms, and their behaviors are influenced by their charge.
Cations are smaller than their parent atoms because they’ve lost an electron, resulting in a stronger pull among the remaining electrons. Anions, on the other hand, are larger than their parent atoms because they’ve gained extra electrons, leading to increased electron repulsion.
Ionic compounds often form crystalline structures with high melting and boiling points. They’re also excellent conductors of electricity when dissolved in water or melted. This is due to the movement of ions, which carry electric charges, facilitating the flow of current.
Real-World Applications: Atoms and Ions at Work
Atoms and ions aren’t just abstract concepts; they’re at the heart of many real-world phenomena and applications.
Atom Applications
Atomic properties play a crucial role in understanding and engineering materials. The arrangement of atoms determines the properties of solids, liquids, and gases. For example, the carbon atoms in diamond form a crystal lattice that gives the gem its remarkable hardness.
Atomic absorption and emission spectra are also essential tools in analytical chemistry. Every element emits and absorbs light at specific wavelengths, creating a unique spectral fingerprint. Scientists can identify elements in unknown substances by analyzing the light they emit or absorb.
Ion Applications
Ions are integral to various fields, from medicine to energy production. One of the most notable applications is in batteries. Rechargeable lithium-ion batteries power our smartphones, laptops, and electric vehicles. These batteries store and release energy through the movement of lithium ions between electrodes.
Ion implantation is another critical process. It involves bombarding materials with high-energy ions to alter their physical and chemical properties. This technique is used in semiconductor manufacturing to create the precise doping needed for electronic components.
Energetic Transformations: Atomic and Ionic States
Atoms and ions are not static entities; they can transition between various energetic states, each with its own significance.
Atomic Excitations
Atoms can absorb energy in the form of photons, causing their electrons to move to higher energy levels. These excited states are temporary, as electrons tend to return to their lower energy levels, releasing the absorbed energy as photons. This phenomenon is the basis of emission and absorption spectroscopy, essential techniques used in studying the composition of materials and even distant stars.
The specific energy levels an atom’s electrons can occupy are dictated by its electron configuration. This configuration determines the element’s chemical properties and reactivity. Excitations play a pivotal role in processes like photosynthesis, where light energy is harnessed by plants to convert into chemical energy.
Ionic Excitations
Ions, too, can undergo energetic changes, but with a twist. Since ions already have unequal numbers of protons and electrons, their energy levels and behavior differ from neutral atoms. The interactions between ions in compounds lead to fascinating phenomena.
Take fluorescent lights as an example. Neon gas, when excited by electricity, emits a brilliant glow. This phenomenon occurs because the electric current provides energy to the neon ions, causing them to transition between energy levels and release photons as they return to their lower states.
Chameleons of the Elements: Different Types of Ions
While the concept of ions might seem straightforward, the world of ions is rich and diverse, encompassing various types with distinct characteristics.
Monoatomic Ions
Monoatomic ions, as the name suggests, are formed from a single atom. They can be either cations or anions, depending on whether they’ve lost or gained electrons. These ions are often vital players in chemical reactions, contributing to the formation of compounds and the balance of charges in solutions.
A familiar example of a monoatomic ion is the sodium cation (Na+), found in table salt and various other compounds. Another is the chloride anion (Cl-), a component of salt and a crucial ion for maintaining the balance of fluids in our bodies.
Polyatomic Ions
Polyatomic ions are more complex structures formed from multiple atoms bonded together and carrying a net charge. These ions are tightly bound and behave as single entities in chemical reactions. They often appear in ionic compounds, and their charges help balance the overall charge of the compound.
An iconic polyatomic ion is the hydroxide ion (OH-), found in solutions of bases like sodium hydroxide (NaOH) and playing a pivotal role in maintaining the pH balance in various systems. Another example is the ammonium ion (NH4+), a component in many fertilizers and industrial processes.
The Quantum Leap: Quantum Mechanics and Electron Behavior
To truly grasp the disparities between atoms and ions, we need to delve into the realm of quantum mechanics, the branch of physics that governs the behavior of particles at the smallest scales.
Atomic Quantum Behavior
At the atomic level, quantum mechanics unveils an array of intriguing phenomena. One of these is the wave-particle duality, which suggests that particles like electrons can exhibit both wave-like and particle-like behaviors. This concept challenges our classical understanding of physics and gives rise to probabilities and uncertainties in predicting particle behavior.
Quantum mechanics also introduces the concept of electron orbitals. Unlike the fixed paths of electrons in the classical model, electrons are found in regions around the nucleus known as orbitals. These regions represent the likelihood of finding an electron in a particular space, rather than a definitive path.
Ionic Quantum Behavior
When electrons are gained or lost, as in ion formation, their quantum behavior changes. Electrons in different energy levels contribute differently to the ion’s overall behavior. This leads to variations in properties such as ion size and reactivity.
Quantum mechanics also governs the interactions between ions in compounds. The arrangement of ions in a crystal lattice, for instance, follows quantum principles, resulting in the distinctive structures and properties of ionic compounds.
Beyond the Periodic Table: Isotopes and Isotonicity
While exploring atoms and ions, we encounter fascinating concepts that branch out into adjacent territories.
Isotopes
Atoms of the same element can have varying numbers of neutrons in their nucleus, resulting in different atomic masses. These variants are known as isotopes. Isotopes of an element have the same number of protons (and thus the same atomic number) but different atomic masses.
Isotopes play essential roles in various fields. Carbon-14, for example, is used in radiocarbon dating to determine the age of archaeological artifacts and ancient remains. Technetium-99m is a crucial isotope used in medical imaging due to its ability to emit gamma rays.
Isotonicity
As we venture further, we encounter the notion of isotonicity, not to be confused with isotopes. In the realm of solutions, an isotonic solution has the same osmotic pressure as another solution. This balance is crucial in contexts such as intravenous fluids in medicine, where isotonic solutions are used to maintain proper hydration and prevent cell damage.
The Unified Force: Electromagnetism and Strong Forces
In the intricate tapestry of the subatomic world, forces are the threads that bind everything together.
Electromagnetic Forces
Atoms and ions, with their charged components, are subject to electromagnetic forces. Oppositely charged particles attract each other, while particles with like charges repel. Electromagnetic forces are behind the formation of chemical bonds, the interactions between charged ions, and even the behavior of electrons within atoms.
These forces determine the stability of atoms and the interactions that give rise to the incredible diversity of compounds we observe in the natural world.
Strong Nuclear Force
At the heart of an atom, protons and neutrons are held together by an immensely powerful force known as the strong nuclear force. This force overcomes the natural electrostatic repulsion between positively charged protons, binding them tightly in the nucleus.
The strong force plays a pivotal role in atomic stability and nuclear reactions, including nuclear fusion that powers the sun and other stars.
FAQs
The fundamental difference lies in their charges and compositions. Atoms are the basic units of matter, composed of protons, neutrons, and electrons, and are electrically neutral. On the other hand, ions are atoms (or molecules) that have lost or gained electrons, resulting in a positive (cation) or negative (anion) charge.
Atoms have a nucleus at their core, comprising protons and neutrons, surrounded by orbiting electrons. Ions have the same nucleus but differ in their electron distribution due to charge. Cations have fewer electrons, while anions have more electrons compared to their parent atoms.
Ions form due to the gain or loss of electrons. Atoms tend to become ions to achieve greater stability by having a complete outer electron shell. Metals tend to lose electrons to become cations, while nonmetals gain electrons to become anions.
Atoms are neutral and have predictable sizes. Ions, however, exhibit charged interactions that influence their properties. Cations are smaller than their parent atoms due to lost electrons, while anions are larger due to gained electrons. Ions are also conductive in solutions due to their ability to carry electric charges.
Atoms’ behaviors in chemical reactions are determined by their electron configurations. Ions, due to their charges, interact through electromagnetic forces, forming bonds that are crucial for compound formation. The charges of ions play a significant role in the nature of these interactions.
Certainly! Atoms’ properties are foundational in materials engineering and analytical chemistry. Ions, however, are pivotal in battery technology, as seen in lithium-ion batteries, and are used in processes like ion implantation in semiconductor manufacturing.
Quantum mechanics governs the behavior of electrons in atoms and ions. Quantum principles influence the arrangement of ions in compounds. Electromagnetic forces drive interactions between charged particles in both atoms and ions, while the strong nuclear force holds protons and neutrons together in atomic nuclei.
Isotopes are variants of the same element with different atomic masses due to varying neutrons. Isotopes find applications in radiocarbon dating and medical imaging. Isotonicity, however, pertains to solutions with equal osmotic pressure and is relevant in contexts like intravenous fluids in medicine.
Atoms and ions are the foundational constituents of matter. They shape the elements around us, influence chemical reactions, and even play roles in celestial phenomena like the behavior of stars. Understanding their differences enriches our comprehension of the universe’s building blocks.
Delve deeper into this captivating subject by continuing to explore reputable educational resources, scientific literature, and online platforms that offer comprehensive insights into the dynamic contrasts between atoms and ions.
Read More:
Contents
- Differences Between Atom and Ion
- Atomic Structure: The Heart of the Matter
- Charged Affairs: Ion Formation
- Properties and Behavior: A Tale of Two Behaviors
- Real-World Applications: Atoms and Ions at Work
- Energetic Transformations: Atomic and Ionic States
- Chameleons of the Elements: Different Types of Ions
- The Quantum Leap: Quantum Mechanics and Electron Behavior
- Beyond the Periodic Table: Isotopes and Isotonicity
- The Unified Force: Electromagnetism and Strong Forces
- FAQs