Chemistry
Welcome to our captivating category page that delves into the intriguing realm of chemistry! Here, you’ll embark on a journey of discovery as we unravel the mysteries behind the differences in terms and other important elements in the field of chemistry. Whether you’re a curious student, an avid learner, or simply someone with an interest in the subject, this collection of content will broaden your understanding and deepen your appreciation for the wonders of chemistry.
-

Diesel vs Petrol
In the world of automotive choices, the age-old debate between petrol and diesel continues to rev its engines. It's a decision that affects not only the way your vehicle performs but also your wallet and the environment. So, let's dive into the fascinating realm of fuel types and explore the nuances that set petrol and diesel apart. At the heart of the petrol vs diesel dilemma are fundamental differences in composition and combustion. Petrol, also known as gasoline, is a highly refined liquid fuel primarily composed of hydrocarbons. It's the spark plug that ignites the controlled explosion within the engine's cylinders, propelling your vehicle forward with a smooth and responsive acceleration. Ideal for city driving and quick starts, petrol offers a refined and efficient ride. On the flip side, diesel fuel boasts a higher energy density, providing better fuel efficiency and longer driving ranges. The compression-ignition process in diesel engines relies on heat generated during compression to ignite the fuel. This unique characteristic results in substantial low-end torque, making diesel engines the go-to choice for long-distance travel, towing heavy loads, and venturing off the beaten path. But the differences don't end there. Maintenance costs, environmental impact, and even the characteristic noise of each engine type play pivotal roles in the petrol vs diesel equation. To make an informed decision for your vehicle, stay tuned as we unravel the intricacies that set these two fuel giants apart. Whether you're a city slicker or an off-road adventurer, the right fuel awaits your choice.
-

Petrol vs Gas
When it comes to fueling your vehicle, the choice between gas and petrol is often determined by regional terminology and measurement units. In the United States, "gasoline" or simply "gas" is the prevalent term, while in many other parts of the world, "petrol" is the preferred word. However, beyond the terminology differences, these fuels are essentially identical, both derived from crude oil and consisting of hydrocarbons. One notable distinction lies in the pricing structure, with gas priced in dollars per gallon in the U.S. and petrol priced in local currency per liter in most other countries. Fuel efficiency is another area where these terms diverge, with gas using miles per gallon (MPG) and petrol employing liters per 100 kilometers (L/100 km) for measurement. Despite these differences, both gas and petrol share common traits in terms of vehicle compatibility and environmental impact. They are compatible with most internal combustion engines and contribute to carbon emissions when burned. Additionally, both fuels are subject to ongoing research and development efforts to reduce their environmental footprint and promote cleaner alternatives. Ultimately, your choice between gas and petrol should consider factors like your geographical location, vehicle compatibility, and budget. Whether you call it "gas" or "petrol," understanding these key differences empowers you to make an informed decision at the pump.
-

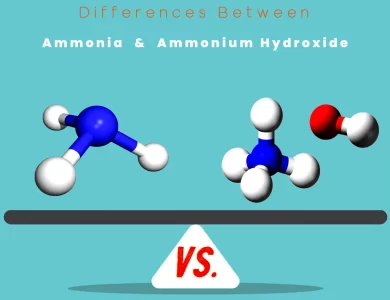
Ammonium Hydroxide vs Ammonia
In the world of chemicals, subtle distinctions can lead to significant differences in properties, applications, and safety considerations. Two such compounds that often cause confusion are ammonia and ammonium hydroxide. While they share a similar pungent odor, they are not the same, and understanding their disparities is crucial, especially when it comes to handling and applications. Ammonia (NH3): This compound is a colorless gas known for its sharp, suffocating smell. It's widely used in various industries, from agriculture as a key component of fertilizers to household cleaning products. Ammonia is also a vital player in industrial refrigeration systems and even finds application in adjusting the pH of solutions. Ammonium Hydroxide (NH4OH): In contrast, ammonium hydroxide is not a standalone compound but rather a liquid solution created by dissolving ammonia gas in water. While it retains that unmistakable ammonia odor, it's in a different physical state. Ammonium hydroxide has diverse applications, from being a cleaning agent in households and industries to serving as a reagent in laboratories. It even plays a role in the food industry for pH adjustment in certain products. Understanding the differences between these two chemicals is not just a matter of semantics but a matter of safety and efficacy in various applications. Whether you encounter them while cleaning your home or in industrial processes, knowing their unique characteristics is essential for making informed decisions and ensuring safe practices.
-

Global Warming vs Ozone Depletion
In the ongoing battle to protect our planet's environment, two critical concerns take the center stage: ozone depletion and global warming. While they may seem interconnected, these environmental issues have unique characteristics that set them apart. Ozone depletion primarily stems from ozone-depleting substances (ODS), leading to the infamous ozone hole and increased UV radiation. Global warming, on the other hand, is driven by greenhouse gas emissions, resulting in rising temperatures, extreme weather events, and broader climate changes. The spatial and temporal scales of these issues differ significantly. Ozone depletion manifests seasonally, with the Antarctic ozone hole as a prime example, while global warming unfolds over decades and affects the entire planet. Both concerns require international cooperation and individual actions to mitigate their impacts. Join us as we delve deeper into the key differences between ozone depletion and global warming, understanding their causes, impacts, and the pivotal role you can play in preserving our planet's future.
-

Polymer vs Resin
In the realm of materials science, the choice between resin and polymer can significantly impact the success of a project. While these terms are sometimes used interchangeably, they refer to distinct classes of materials with unique characteristics and applications. Resin encompasses a diverse range of materials, including both natural and synthetic compounds. What sets resins apart is their adhesive nature and versatility in coatings and encapsulation. They can be found in everything from woodworking adhesives to the protective coatings on your smartphone screen. Resins are often praised for their chemical resistance and thermal stability, making them valuable in various industrial settings. Polymers, on the other hand, are a more extensive category of materials characterized by the repetition of monomer units. This repeating structure grants polymers their remarkable diversity, from everyday plastics like polyethylene to high-strength composites used in aerospace. Polymers excel in applications where flexibility, lightweight properties, and elasticity are crucial. Intriguingly, some polymers can also function as resins when adhesive or coating properties are required, blurring the lines between these two material classes. Therefore, understanding their differences is paramount in selecting the right material for specific tasks, whether it's crafting artful creations or engineering cutting-edge technologies.
-

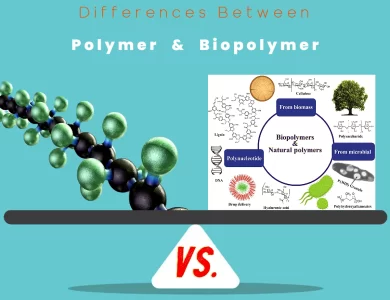
Biopolymer vs Polymer
In the ever-evolving realm of materials science, the distinction between polymers and biopolymers is a pivotal one. These substances, although related by name, possess vastly different characteristics, sources, and environmental impacts. Understanding these differences is not only essential for scientists and engineers but also for anyone interested in the sustainable future of our planet. Polymers, often synonymous with synthetic plastics, are derived from petrochemicals through intricate chemical processes. These versatile materials have become ubiquitous in our daily lives, finding applications in countless industries. From the plastic bottles that hold our beverages to the insulation in our homes, polymers are the workhorses of modern manufacturing. However, their dominance comes at a price - their environmental footprint. Many synthetic polymers are non-biodegradable, contributing to long-lasting pollution and resource depletion. Biopolymers, on the other hand, are the natural counterparts. These materials, as the name suggests, originate from living organisms. They include substances like cellulose, found in plant cell walls, and DNA, the genetic code of all life forms. What sets biopolymers apart is their sustainable nature. They are often derived from renewable biological sources and possess the remarkable ability to biodegrade, returning to the Earth without leaving a lasting mark. This eco-friendliness has fueled their adoption in various eco-conscious industries, such as biodegradable packaging and regenerative medicine. As we venture deeper into the realm of polymers and biopolymers, we'll uncover their composition, sources, properties, and applications. We'll also explore the environmental impact, recycling challenges, cost considerations, and regulatory aspects that shape these materials' roles in our world. Join us on this enlightening journey to grasp the "Differences Between Polymer vs Biopolymer" and their significance in the grand tapestry of materials science.
-

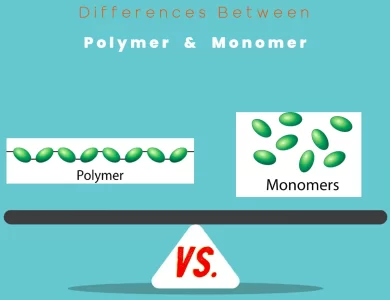
Monomer vs Polymer
Polymers and monomers may sound like scientific jargon, but they play pivotal roles in our daily lives. At their core, these terms encapsulate the fundamental building blocks of matter, with unique properties and applications. In our journey to comprehend the distinctions between polymers and monomers, we delve into their molecular structures, chemical properties, and real-world significance. Polymers, those long chains of repeating monomer units, are the unsung heroes of modernity. From the synthetic fibers in our clothes to the plastic containers that store our food, polymers are everywhere. They offer versatility that can be tailored to meet specific needs, making them the backbone of various industries. Monomers, on the other hand, are the precursors, the individual molecules that join together to form polymers. Think of them as the bricks in the construction of molecular architecture. While smaller in size, monomers wield precision and control in chemical synthesis, allowing scientists and engineers to craft materials with exacting specifications. In this guide, we explore the key differences between polymers and monomers, shedding light on their distinct roles in chemistry, industry, and our evolving understanding of materials. From their size and structure to their diverse applications and environmental impacts, we navigate the fascinating world of these chemical entities. Join us as we embark on an enlightening journey to unravel the mysteries of Polymer vs Monomer.
-

Vinyl vs PVC
In the realm of construction and interior design, the choice of materials can make or break a project's success. Two commonly confused materials, PVC (Polyvinyl Chloride) and Vinyl, each bring their unique qualities to the table. To clarify the distinctions and help you make informed decisions for your next endeavor, let's delve into the key differences between PVC vs Vinyl. Material Composition: The primary difference between PVC and Vinyl lies in their material composition. PVC is a synthetic polymer made from vinyl chloride monomers, available in both rigid and flexible forms. Rigid PVC, often referred to as uPVC, is sturdy and used in applications like plumbing and window frames. In contrast, flexible PVC contains plasticizers that make it pliable and ideal for vinyl flooring and medical tubing. On the other hand, Vinyl, specifically PVC-based vinyl, shares the same PVC resin base but is inherently flexible, making it a go-to choice for interior design elements like flooring, upholstery, and wall coverings. Applications: The applications of PVC and Vinyl differ significantly due to their flexibility and durability attributes. PVC is often employed in structural and industrial applications where rigidity and resistance to corrosion are paramount. This includes water pipes, electrical conduits, and window frames. In contrast, Vinyl, with its inherent flexibility, is a preferred choice for interior design projects. It excels in applications such as luxury vinyl flooring (LVP), vinyl upholstery, banners, decals, and more. Vinyl offers a broad spectrum of design options, mimicking the appearance of natural materials like wood and stone, making it a favorite among interior designers and homeowners alike.
-

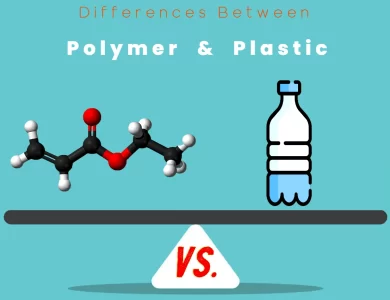
Plastic vs Polymer
In the vast landscape of materials that shape our world, polymers and plastics stand as two prominent players. While these terms are often used interchangeably, they are not synonymous. Understanding the differences between polymers and plastics is not only fascinating but also essential in today's environmentally conscious era. Composition and Structure: Polymers are a diverse class of materials comprising both natural and synthetic substances. They consist of large molecules formed by repeating structural units, known as monomers. These monomers are chemically bonded together, creating long chains or networks. On the other hand, plastics are a subset of synthetic polymers, specifically chosen for their ability to be molded when exposed to heat or pressure. They are typically derived from petrochemicals through a process called polymerization. Properties: Polymers exhibit a wide range of properties, from flexibility and strength to varying thermal characteristics. Their properties depend on factors like the type of monomers and their arrangement. Plastics, as synthetic polymers, inherit these properties but often have added moldability due to their specific composition. Applications: Polymers find applications in numerous industries, including medical, construction, automotive, and electronics. In contrast, plastics, with their moldable nature, are ubiquitous in packaging, consumer goods, and agriculture, among others. Environmental Impact: One of the most critical distinctions lies in their environmental impact. While some polymers, especially natural ones, can be biodegradable, many synthetic polymers, including traditional plastics, are non-biodegradable and contribute to environmental challenges like plastic pollution.
-

Aluminum vs Alloy
Are you facing the dilemma of choosing between alloy and aluminum for your next project? This decision can significantly impact the outcome, whether you're constructing a towering skyscraper, designing an efficient aerospace component, or even selecting the perfect cookware for your kitchen. To make an informed choice, let's delve into the differences between these two materials. Alloy, in essence, is an amalgamation of two or more elements, often including metals and sometimes non-metals, meticulously engineered to exhibit specific properties. These properties can range from remarkable strength and durability to superior corrosion resistance and electrical conductivity. Alloys span a broad spectrum, encompassing stalwarts like steel, brass, bronze, and high-strength alloys. Each alloy type boasts unique characteristics, making them suitable for diverse industries and applications. Aluminum, on the other hand, is a lightweight metal, denoted by the symbol Al, naturally occurring in the Earth's crust. It stands out for its low density, rendering it about one-third as heavy as steel. This characteristic makes aluminum a favored choice in industries where weight reduction is paramount, such as aerospace and automotive. Yet, aluminum's appeal extends beyond its lightweight nature; it boasts excellent thermal and electrical conductivity and corrosion resistance, further enhancing its versatility.