Chemistry
Welcome to our captivating category page that delves into the intriguing realm of chemistry! Here, you’ll embark on a journey of discovery as we unravel the mysteries behind the differences in terms and other important elements in the field of chemistry. Whether you’re a curious student, an avid learner, or simply someone with an interest in the subject, this collection of content will broaden your understanding and deepen your appreciation for the wonders of chemistry.
-

Aragonite vs Calcite
Calcite and Aragonite, both fascinating minerals with a shared composition of calcium carbonate (CaCO3), hold distinct positions in the world of geology. These mineral siblings captivate scientists, collectors, and environmentalists alike, offering unique characteristics and ecological roles that set them apart. One of the primary distinctions lies in their crystal structures. Calcite, belonging to the trigonal crystal system, exhibits a rhombohedral shape and a vibrant spectrum of colors. It boasts optical anomalies like double refraction and reacts strongly with acids. In contrast, Aragonite's orthorhombic structure results in prismatic or needle-like crystals. It tends to be colorless or pale and lacks double refraction. Their hardness levels also differ, with Calcite measuring around 3 on the Mohs scale and Aragonite being slightly harder at 3.5 to 4. Additionally, Calcite showcases versatile applications in construction, agriculture, and optics, while Aragonite's significance lies in its role as the primary component of coral reefs, making it vital for marine ecosystems and a focal point in ocean acidification studies. Explore the captivating world of Calcite and Aragonite as we delve into their unique properties, formation processes, and environmental significance. These minerals, though similar in composition, offer distinct windows into the intricate workings of our planet's geological and ecological systems.
-

Clarifier vs Purifier
Are you intrigued by the world of industrial equipment and the intricacies of liquid processing? If so, you're about to embark on a journey that unveils the fundamental distinctions between two remarkable machines: purifiers and clarifiers. These unsung heroes of various industries wield unique capabilities, and understanding their differences is key to choosing the right one for your specific needs. Purifiers are precision instruments designed to elevate liquid quality by eliminating impurities such as solid particles and water. They achieve this through the powerful force of centrifugation, making them ideal for industries like marine, oil refining, and precision manufacturing, where the purity of fluids is paramount. Purifiers excel at meticulous separation, ensuring the highest liquid quality possible. Clarifiers, on the other hand, are specialists in solid-liquid separation. Operating on the principle of gravity settling, they efficiently remove solids from liquids. Clarifiers find their niche in wastewater treatment, mining, agriculture, and other industries where efficient separation is the primary goal. Their simplicity in design and minimal maintenance requirements make them a cost-effective choice. As you delve deeper into the differences between purifiers and clarifiers, you'll gain insights into their distinct applications, design complexities, maintenance needs, and cost considerations. So, whether you prioritize liquid quality enhancement or efficient solid-liquid separation, this exploration will guide you toward the right choice for your liquid processing endeavors.
-

Potassium Iodide vs Iodine
In the realm of chemistry and health, understanding the differences between iodine and potassium iodide is essential. While both compounds contain the crucial element iodine, they serve distinct roles with unique properties and applications. Iodine, symbolized as I on the periodic table, exists as a purple-black diatomic molecule with a relatively low boiling point. It can be found in seaweed and seafood, making it a dietary essential. Iodine is renowned for its applications in medical antiseptics, dietary supplements, chemical reactions, and even photography, where it contributes to the creation of light-sensitive emulsions. On the other hand, potassium iodide, often abbreviated as KI, is a white crystalline salt, primarily produced synthetically. Its critical role lies in radiation protection during emergencies, safeguarding the thyroid gland from harmful radioactive iodine isotopes. It's also used in pharmaceuticals and plays a crucial part in the iodization of table salt to combat iodine deficiency disorders. In this comprehensive guide, we delve into the chemical properties, health benefits, and potential risks associated with iodine and potassium iodide, shedding light on when and why you might encounter these compounds in your everyday life. So, if you're curious about the distinctions that set these two compounds apart, read on to unravel the captivating world of "Differences Between Iodine vs Potassium Iodide."
-

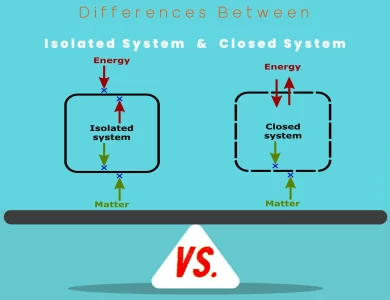
Closed System vs Isolated System
In the realm of physics and thermodynamics, two fundamental concepts—isolated systems and closed systems—play a pivotal role in understanding how matter and energy interact within physical boundaries. The primary disparity between these two lies in their treatment of energy exchange. Isolated systems, often encountered in theoretical physics, are characterized by their strict conservation of both matter and energy. These systems do not permit any matter or energy exchange with the surrounding environment, making them valuable for theoretical studies and idealized models. In contrast, closed systems, commonly encountered in practical applications, allow energy transfer across their boundaries while preserving a constant mass. This flexibility makes them indispensable for understanding real-world processes like chemical reactions, engineering systems, and environmental phenomena, where energy interactions play a vital role. By exploring the nuances of isolated and closed systems, scientists, engineers, and researchers can make informed decisions on which framework to apply based on the specific goals and nature of the system under study. This knowledge is crucial for both theoretical explorations of fundamental principles and the optimization of practical processes in various fields.
-

Iodide vs Iodine
Iodine and iodide, though often used interchangeably, are distinct chemical entities with vital roles in various domains. Iodine, represented as I2, is a diatomic molecule known for its distinctive violet-black crystals and a pungent odor. In contrast, iodide exists as an ion, denoted as I-, typically found in the form of white crystalline salts. These two compounds might appear similar, but their differences are far from subtle. Iodine is crucial for thyroid health, playing a pivotal role in the synthesis of thyroid hormones essential for metabolism regulation. It finds applications in disinfection, chemistry, and even nuclear medicine, where radioactive iodine isotopes aid in diagnostics and therapy. On the other hand, iodide, commonly as potassium iodide (KI), is used in pharmaceuticals to treat respiratory conditions and for radiation protection during nuclear emergencies. It's also a valuable component in analytical chemistry, facilitating redox titrations and precipitation reactions. Understanding the disparities between iodine and iodide empowers us to make informed choices, whether in our health journey, laboratory experiments, or even when preparing for potential environmental contingencies. Join us on this illuminating exploration of iodine and iodide, where we unravel their unique properties and applications, paving the way for a deeper understanding of these essential chemical companions.
-

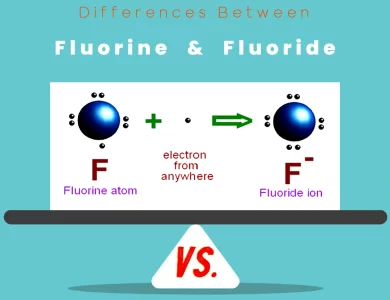
Fluoride vs Fluorine
In the realm of chemistry, the terms "fluorine" and "fluoride" may sound deceptively similar, but their disparities are profound. Fluorine, with its atomic symbol F and atomic number 9, is a fiercely reactive element. It exists as a diatomic gas, rarely found in nature in its elemental form. Its extreme reactivity makes it one of the most formidable elements in the periodic table. In contrast, fluoride is not an element but a chemical compound. It contains the fluoride ion (F⁻) and is formed when fluorine reacts with other elements. Fluoride compounds can be solids, liquids, or gases, depending on their composition. They are notably less reactive than their parent element, making them invaluable in various industries and dental care. The distinctions between these two entities extend beyond their nature. Fluorine, with its pale yellow-green gas and acrid odor, is notoriously toxic and not used directly in most applications. Fluoride compounds, on the other hand, are harnessed for their stability and versatility. They find applications in water treatment, metallurgy, and even toothpaste, where they safeguard our dental health by fortifying tooth enamel. However, the environmental impact of fluoride and the careful regulation of its levels in drinking water remain subjects of ongoing debate and study. To delve deeper into the intriguing world of fluorine and fluoride, read on and explore their diverse roles, properties, and applications.
-

Nucleophile vs Base
In the fascinating world of chemistry, understanding the distinctions between a base and a nucleophile is paramount. Bases, known for their role in acid-base reactions, have the unique ability to accept protons or donate lone pair electrons, thus influencing the pH of a solution. Common examples include hydroxide ions (OH-) and ammonia (NH3). In contrast, nucleophiles, electron-rich entities, are skilled in attacking electrophiles, leading to the formation of new covalent bonds. Prominent nucleophiles encompass chloride ions (Cl-), cyanide ions (CN-), and various amines. These disparities are crucial in comprehending their functions within chemical reactions, making the base vs. nucleophile distinction an essential concept in the realm of chemistry. Whether you're a budding chemist or simply curious about the scientific intricacies of molecules, delving into these differences unveils a world of insights into the dynamic processes that surround us.
-

Nuclear Bomb vs Nuclear Reactor
In the realm of nuclear science and technology, two entities stand in stark contrast: the nuclear reactor and the nuclear bomb. While they both involve the release of nuclear energy, their purposes, functions, and implications couldn't be more different. Nuclear Reactor: A nuclear reactor is a remarkable piece of engineering designed with a singular, constructive aim – to generate electricity. These reactors are integral to the global energy infrastructure, providing a substantial portion of the world's electricity through controlled nuclear reactions. They operate by harnessing the heat generated from nuclear fission, a process where the nucleus of an atom is split into smaller fragments. This controlled chain reaction releases a tremendous amount of heat, which is then used to produce steam. The steam powers turbines, ultimately generating electricity. The consequences of a well-maintained and safely operated nuclear reactor are largely positive, including clean energy production with minimal greenhouse gas emissions. However, they do produce radioactive waste, which requires careful management and disposal. Nuclear Bomb: In stark contrast to nuclear reactors, nuclear bombs are instruments of destruction. Their sole purpose is to unleash an enormous amount of energy in the form of a nuclear explosion, resulting in mass devastation and loss of life. Nuclear bombs operate on the principles of uncontrolled nuclear chain reactions, rapidly releasing the energy stored in fissile materials. This release leads to a cataclysmic blast, accompanied by intense heat, radiation, and the infamous mushroom cloud. The consequences of a nuclear bomb are overwhelmingly negative, causing widespread death, destruction of infrastructure, and long-lasting environmental damage. The release of radiation can result in severe health issues for survivors and contaminate large areas for extended periods, leaving a haunting legacy. Understanding the fundamental disparities between these two powerful technologies is not only an exercise in knowledge but also a critical…
-

Net Zero vs Carbon Neutral
In the realm of environmental sustainability, two terms often surface prominently: Carbon Neutral and Net Zero. While they both revolve around the overarching goal of reducing greenhouse gas emissions, they approach it in distinctive ways. Carbon Neutral signifies achieving a delicate equilibrium where the total emissions generated are counterbalanced by emissions removed from the atmosphere. The journey to carbon neutrality encompasses reducing emissions as much as possible through energy efficiency measures, sustainable practices, and responsible resource management. What sets it apart is the inclusion of carbon offsetting, a process through which any remaining emissions are compensated for by investing in projects that capture or reduce an equivalent amount of greenhouse gases elsewhere. This approach is pragmatic, providing a practical means for individuals, businesses, and nations to take immediate action toward climate responsibility. Net Zero, on the other hand, sets a more ambitious goalpost. It not only balances emissions but also strives to minimize emissions to the lowest achievable level. Achieving Net Zero entails aggressive emission reduction measures like transitioning to renewable energy, electrifying transportation, and enhancing energy efficiency. Moreover, it encompasses carbon removal technologies like direct air capture and carbon capture and storage (CCS), along with bolstering natural carbon sinks such as forests. Net Zero is a long-term commitment that involves substantial investments and a comprehensive approach to emissions reduction. So, whether you opt for the pragmatic path of Carbon Neutrality or embark on the ambitious journey toward Net Zero emissions, understanding these differences is crucial in charting a sustainable course for the future.
-

Pig Iron vs Sponge Iron
In the realm of iron production, two distinct players take center stage: Sponge Iron and Pig Iron. While they both share the common element of iron, the differences between them are profound and consequential. Let's delve into the intricacies of Sponge Iron vs Pig Iron and uncover the key distinctions that set them apart. Production Process: The primary disparity lies in their production processes. Sponge Iron is crafted through direct reduction, a method that involves reducing iron ore using a reducing gas or solid reductant at lower temperatures. This results in a porous, sponge-like structure, giving it its name. In contrast, Pig Iron is born in the fiery depths of a blast furnace, where iron ore is smelted with coke and limestone. This process yields a dense, carbon-rich material. Chemical Composition: Chemically, they diverge significantly. Sponge Iron is celebrated for its low carbon content, typically below 0.1%, and minimal impurities. Pig Iron, on the other hand, boasts a higher carbon content, approximately 3-4%, and contains variable impurities like silicon, phosphorus, and sulfur. Applications: Sponge Iron finds its niche in applications requiring clean, low-carbon iron, such as steel production and powder metallurgy. Pig Iron, with its high carbon and impurities, is best suited for cast iron production, foundry work, and certain steelmaking processes. Environmental Impact: When considering environmental factors, Sponge Iron gets the green nod. Its production generates lower carbon emissions and is more energy-efficient compared to the energy-intensive process of producing Pig Iron. The choice between Sponge Iron and Pig Iron boils down to your specific needs, priorities, and sustainability goals. Each has its merits, and understanding their differences is pivotal in making informed decisions in the world of iron production.