Chemistry
Welcome to our captivating category page that delves into the intriguing realm of chemistry! Here, you’ll embark on a journey of discovery as we unravel the mysteries behind the differences in terms and other important elements in the field of chemistry. Whether you’re a curious student, an avid learner, or simply someone with an interest in the subject, this collection of content will broaden your understanding and deepen your appreciation for the wonders of chemistry.
-

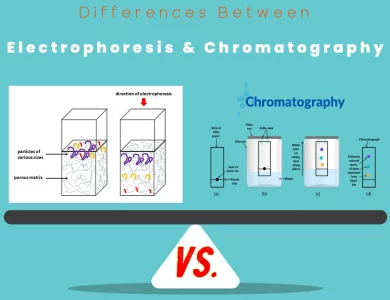
Chromatography vs Electrophoresis
In the realm of analytical chemistry, the choice between electrophoresis and chromatography can be akin to selecting the right tool from a scientist's treasure chest. These two techniques stand as pillars in the world of molecular analysis, each offering a unique set of capabilities that cater to diverse scientific needs. Electrophoresis, often associated with the graceful migration of DNA bands on a gel, operates on the fundamental principle of charged particle movement in an electric field. This technique is a powerhouse for the separation of charged molecules, including DNA, RNA, proteins, and peptides. It excels at resolving biomolecules based on their size, shape, and charge, making it indispensable in molecular biology, biochemistry, and forensic science. On the other hand, chromatography is like a chameleon in the laboratory, adapting to an extensive range of analytes, both charged and uncharged. Whether you're working with small organic compounds, large biomolecules, or gases, chromatography has you covered. Its magic lies in the differential distribution of compounds between a stationary phase and a mobile phase, guided by properties such as polarity, size, and affinity. This versatility makes chromatography a cornerstone in fields spanning pharmaceuticals, environmental analysis, food science, and beyond.
-

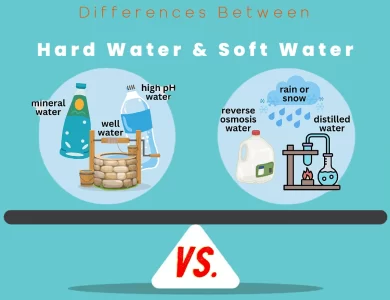
Soft Water vs Hard Water
Water is an essential element in our daily lives, but it comes in two distinct flavors: hard water and soft water. Understanding the differences between them can significantly impact your household, personal well-being, and even your environmental footprint. Hard Water: This type of water is characterized by its high mineral content, primarily calcium and magnesium ions. These minerals find their way into the water as it percolates through rock and soil layers, picking up various substances along the way. The hardness of water is typically measured in parts per million (ppm) or grains per gallon (gpg) of calcium carbonate (CaCO3) equivalents. The consequences of hard water are many. It can lead to scale buildup in plumbing fixtures and appliances, making them less efficient and potentially causing maintenance headaches. Hard water can also affect your skin and hair, leaving them dry and dull. Additionally, it may influence the taste of your food and beverages, as well as the ease of lathering with soap. Soft Water: In contrast, soft water contains fewer dissolved minerals, primarily calcium and magnesium ions. Soft water is often naturally occurring in areas with specific geological formations or can be produced through water softening methods. It's prized for its ability to lather easily with soap and detergents, leaving no scale or residue behind. Soft water is also kinder to your skin and hair, resulting in a moisturizing effect and vibrant hair.
-

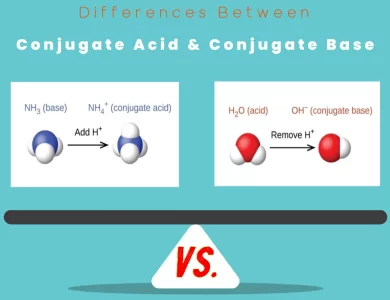
Conjugate Base vs Conjugate Acid
Acid-base chemistry is the backbone of countless chemical reactions, from the laboratory to the natural world. At its heart lie two critical players: conjugate acids and conjugate bases. Understanding their differences is akin to unlocking the secrets of chemical reactivity and the behaviors of substances in diverse environments. Conjugate Acid: Think of this as the "donor" in a chemical tango. Conjugate acids are formed when a base accepts a proton (H+). This proton donation transforms the base into its conjugate acid, changing its chemical character and often rendering it positively charged. In practical terms, conjugate acids increase the acidity of a solution and play pivotal roles in defining the pH levels of substances. Conjugate Base: On the flip side, conjugate bases are like the gracious "receivers" in the chemical dance. They form when an acid donates a proton (H+), resulting in the base's transformation into its conjugate base, typically carrying a negative charge. Conjugate bases tend to elevate the basicity of a solution and are essential players in maintaining chemical equilibrium.
-

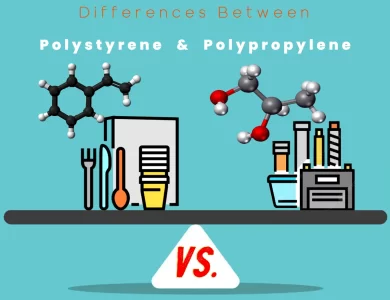
Polypropylene vs Polystyrene
In the world of plastics, Polystyrene (PS) and Polypropylene (PP) are two prominent players, each with its unique set of characteristics and applications. Understanding the differences between these materials is crucial for selecting the right one for your specific needs. Chemical Composition: Polystyrene is derived from styrene monomers, featuring a benzene ring in its chemical structure, imparting transparency and brittleness. On the other hand, Polypropylene is synthesized from propylene monomers, forming a linear carbon and hydrogen arrangement, resulting in superior heat resistance and toughness. Physical Properties: Polystyrene is known for its transparency, lower density, and limited impact resistance, making it ideal for applications requiring clarity, such as disposable tableware and CD cases. In contrast, Polypropylene offers excellent heat resistance, higher impact resistance, and superior chemical resistance, making it suitable for items like food packaging, automotive parts, and medical devices. Environmental Impact: While neither of these plastics is biodegradable, Polypropylene is often considered more environmentally friendly due to its better recyclability. Cost: Polystyrene is generally more cost-effective, making it a popular choice for disposable and low-cost products, while Polypropylene's cost can vary based on factors like grade and application. Understanding the nuances between Polystyrene and Polypropylene empowers you to make informed decisions, whether you're designing sustainable packaging, engineering durable components, or considering the environmental footprint of your materials.
-

Plastic vs Polycarbonate
In the world of materials, the choice between Polycarbonate and Plastic often plays a pivotal role in the success of a project. While both fall under the category of polymers, these materials differ significantly in terms of composition, properties, and applications. Polycarbonate, known for its exceptional transparency and strength, is derived from repeating carbonates and offers superb impact resistance. It is the material of choice for applications where optical clarity and durability are paramount, such as safety equipment, optical lenses, and aerospace components. Plastic, on the other hand, is a broad term encompassing a diverse range of synthetic polymers, each with its unique chemical composition and characteristics. Plastics vary in transparency, strength, heat resistance, and chemical compatibility, making them suitable for a wide array of applications, including packaging, automotive components, and medical devices. To make an informed choice between Polycarbonate and Plastic, it's essential to consider your project's specific requirements, budget constraints, and environmental goals. Whether you prioritize optical clarity, cost-effectiveness, or sustainability, understanding the differences between these materials empowers you to select the one that aligns perfectly with your needs. Explore the nuances, and navigate the world of materials confidently.
-

Fire Point vs Flash Point
In the realm of fire safety and the handling of flammable substances, understanding the differences between Flash Point and Fire Point is paramount. These two terms are often used interchangeably, but they serve distinct purposes in assessing the flammability of materials. Flash Point is the temperature at which a liquid substance produces enough vapor to ignite when exposed to an open flame or spark. It essentially marks the ignition threshold and is crucial for industries involved in the transportation, storage, and handling of flammable liquids. Knowing the flash point of a substance helps prevent accidents, guides safe storage practices, and ensures compliance with safety regulations. On the other hand, Fire Point represents the temperature at which a liquid substance can sustain combustion once ignited. It goes beyond the initial ignition and tells us whether a fire will continue to burn without the need for an external ignition source. Fire point is particularly significant in assessing the severity of a fire hazard and planning effective firefighting strategies and emergency response protocols. To gain a comprehensive understanding of these critical parameters, it's essential to delve into their testing methods, regulatory implications, and their roles in various industries. By recognizing the distinctions between flash point and fire point, industries can enhance safety measures, mitigate fire risks, and ensure the secure handling of flammable materials.
-

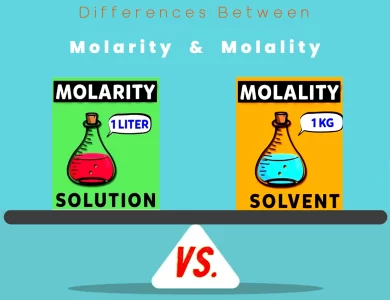
Molality vs Molarity
In the captivating realm of chemistry, two terms often steal the limelight: Molarity and Molality. These two fundamental concepts are pivotal for understanding the concentration of solutions, yet they diverge in significant ways. Molarity, represented as 'M,' quantifies the number of moles of solute per liter of solution and is widely employed in various industries. However, it's worth noting that Molarity is temperature-sensitive due to its reliance on the volume of the solution. On the other hand, Molality, symbolized as 'm,' measures the moles of solute per kilogram of solvent. What sets it apart is its temperature-independence. Molality is favored in scenarios where temperature fluctuations play a critical role, such as in cryogenics or specific chemical reactions. This exploration into the differences between Molarity and Molality will equip you with the knowledge to make informed decisions in the lab or industry, ensuring precise concentration control in your scientific endeavors.
-

Oxyacids vs Binary Acids
In the realm of geology, two fundamental processes, cementation and compaction, stand as the cornerstone of sedimentary rock formation. While they share the common objective of transforming loose sediments into solid rock, their mechanisms and impacts diverge significantly. Cementation, akin to nature's adhesive, is a post-depositional process where minerals act as binding agents. These minerals precipitate from mineral-rich solutions, gluing sediment particles together and solidifying them over time. Picture calcite, silica, or iron oxides acting as nature's glue, holding the grains of sand, silt, or clay together. The result? Sedimentary rocks with distinct textures, colors, and porosities, depending on the minerals involved. Compaction, on the other hand, is a physical process intertwined with sediment deposition. It occurs concurrently as layers of sediment accumulate, and the sheer weight of overlying material compresses the sediment below. The particles get squeezed closer together, reducing pore spaces and leading to fine-grained, compacted rocks. Think of it as Earth's slow but persistent sculptor, shaping the sediments into dense, often smooth-textured formations. Understanding these processes is essential for deciphering Earth's history, environmental conditions, and the formation of various sedimentary rock types. So, whether you're a geology enthusiast or a curious mind, delving into the contrasts between cementation and compaction promises a deeper appreciation of our planet's geological wonders.
-

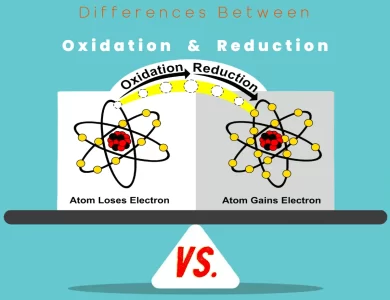
Reduction vs Oxidation
In the captivating realm of chemistry, two fundamental processes, oxidation and reduction, play a pivotal role in shaping the way substances transform and react. Understanding these processes is like unlocking the secrets of chemical reactions, from the rusting of iron to the energy production within our cells. Oxidation is the process where an atom, ion, or molecule loses electrons, leading to an increase in its oxidation state. It's often associated with the addition of oxygen or the removal of hydrogen atoms. Think of it as a substance's willingness to "donate" electrons to other participants in a chemical reaction. On the flip side, reduction is the process where an atom, ion, or molecule gains electrons, causing a decrease in its oxidation state. This can involve the addition of hydrogen or the removal of oxygen. Reduction is akin to a substance's ability to "accept" electrons from other participants in a chemical reaction. These two processes are intricately linked in what we call redox (reduction-oxidation) reactions. In a redox reaction, one substance gets oxidized, losing electrons, while another gets reduced, gaining those electrons. This transfer of electrons is the driving force behind countless chemical reactions, both in the lab and in our daily lives. To gain a deeper understanding of the key distinctions between oxidation and reduction, their roles in chemistry, and their impact on our world, read on in our comprehensive guide. Whether you're a chemistry enthusiast or simply curious about the science that surrounds us, this exploration will shed light on the fascinating world of redox reactions.
-

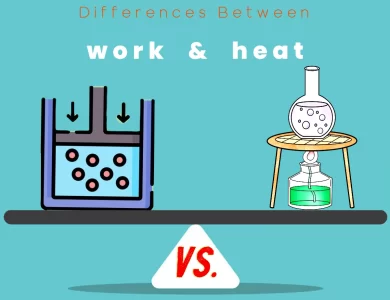
Heat vs Work
Work and heat, though both terms closely related to energy transfer, stand as distinctive concepts in the realm of physics and thermodynamics. These fundamental principles are integral to understanding the flow of energy in various systems and processes. Work, often associated with the application of force, embodies the transfer of mechanical energy leading to the displacement of an object. Its measurement unit, the joule (J), quantifies the extent of this energy transfer. Work's directionality further categorizes it as positive, negative, or zero, dependent on the alignment of force with motion. This concept finds practical application in everything from lifting objects to driving machines. In contrast, heat represents the transfer of thermal energy arising from temperature differences. Like work, it is quantified in joules (J) and finds utility in everyday life, from cooking to temperature regulation. Heat's remarkable feature is its lack of directional component—instead, it focuses solely on the transfer of internal energy through conduction, convection, and radiation. These disparities culminate in a deeper understanding of the First Law of Thermodynamics, which emphasizes energy conservation. Work and heat play pivotal roles in this law, showcasing their relevance in the world of physics and engineering. As we explore the intricate differences between these energy-transfer mechanisms, we gain insight into the forces that shape our physical universe.