| Property | Bases | Nucleophiles |
|---|---|---|
| Role in Reactions | Accept protons or donate lone pair electrons | Attack electrophiles to form new bonds |
| Electron Density | May or may not be electron-rich | Electron-rich |
| Effect on pH | Raises pH by forming hydroxide ions | No direct impact on pH |
| Typical Examples | Hydroxide ions (OH-), ammonia (NH3) | Chloride ions (Cl-), cyanide ions (CN-), amines |
| Common Reactions | Acid-base reactions | Nucleophilic substitution and addition |
| Sensory Properties | Slippery feel and bitter taste (some) | No distinctive sensory properties |
| Strength | Can be strong or weak | Varies in nucleophilicity |
| Definition | Can be defined by Bronsted-Lowry or Lewis theory | Typically defined by their nucleophilic nature |
| Lewis Base vs. Bronsted-Lowry Base | May or may not align with Lewis base definition | May align with Lewis base definition if electron-rich |
| Nucleophilicity | Not applicable | Highly relevant, varies with different nucleophiles |
| Solvent Effects | Influence reactivity in polar solvents | Influence reactivity; polar solvents enhance it |
| pKa Considerations | pKa values relevant for predicting behavior | Not applicable for prediction |
| Leaving Group Impact | Not directly related | Leaving group choice influences reactivity |
Beyond the basics, we’ll uncover the intricacies of how these compounds behave in reactions, their real-world applications, and even peer into the horizon of future developments. Whether you’re a budding chemist eager to expand your knowledge or simply curious about the science that surrounds us, this blog promises to illuminate the fascinating contrasts between bases and nucleophiles.
Differences Between Base and Nucleophile
The main differences between Base and Nucleophile lie in their roles and reactivities. Bases primarily accept protons or donate lone pair electrons in acid-base reactions, raising the pH of a solution. Common examples include hydroxide ions (OH-) and ammonia (NH3). In contrast, nucleophiles are electron-rich species that seek to attack electrophiles, forming new covalent bonds in processes like nucleophilic substitution and addition. Key nucleophiles encompass chloride ions (Cl-), cyanide ions (CN-), and amines. These distinctions are vital in understanding their respective functions in chemical reactions, making Base vs. Nucleophile an essential concept in the world of chemistry.
1. Definitions
Bases
Bases are substances that can accept a proton (H+) or donate a pair of electrons to form a new chemical bond. They are often characterized by their ability to neutralize acids and raise the pH of a solution. Bases are essential in acid-base reactions, where they combine with acidic protons to form water or other products. Common examples of bases include hydroxide ions (OH-), ammonia (NH3), and alkoxides (RO-).
Nucleophiles
On the other hand, nucleophiles are substances that are rich in electrons and seek positively charged nuclei to form new bonds. Nucleophiles are known for their affinity for electron-deficient species, such as electrophiles. They play a crucial role in nucleophilic substitution and addition reactions. Nucleophiles can attack electrophilic centers, leading to bond formation. Common examples of nucleophiles include chloride ions (Cl-), cyanide ions (CN-), and amines (RNH2).
2. Properties of Bases
Now that we have clear definitions of bases and nucleophiles, let’s delve into the properties that define bases:
2.1. Ability to Accept Protons
Bases have the ability to accept protons (H+ ions) from other substances. When they do so, they form a new chemical bond by sharing the proton. This property allows them to neutralize acids, making solutions less acidic.
2.2. Presence of Lone Pair Electrons
Bases typically have lone pair electrons that can be donated to form a new bond. These electrons are often found on atoms like oxygen (O) and nitrogen (N), which are electronegative and can hold onto electrons tightly.
2.3. Higher pH
When bases are added to a solution, they raise the pH, making it more alkaline. This is because the formation of hydroxide ions (OH-) from the base leads to an increase in the concentration of hydroxide ions, which in turn increases the pH.
2.4. Slippery Feel and Bitter Taste
Some common bases, such as sodium hydroxide (NaOH), have a slippery feel and a bitter taste. These sensory properties can help identify the presence of bases in everyday substances.
2.5. Strong vs. Weak Bases
Bases can vary in strength, with some being strong and others weak. Strong bases, like sodium hydroxide, completely dissociate in water and can readily accept protons. Weak bases, on the other hand, only partially dissociate and have a limited ability to accept protons.
3. Properties of Nucleophiles
Now, let’s turn our attention to the defining properties of nucleophiles:
3.1. Electron-Rich Species
Nucleophiles are electron-rich species, meaning they have an excess of electrons compared to the atoms they are reacting with. This electron-rich nature allows them to attack electron-deficient species.
3.2. High Nucleophilicity
The nucleophilicity of a species depends on its electron density and steric hindrance. Highly nucleophilic species are more reactive and have a greater tendency to participate in nucleophilic reactions.
3.3. Attack on Electrophiles
Nucleophiles are known for their ability to attack electrophiles, which are electron-deficient species that have a positive charge or partial positive charge. Nucleophiles share their electrons with electrophiles, forming new covalent bonds.
3.4. Solvent Effects
The choice of solvent can influence the reactivity of nucleophiles. Polar solvents tend to stabilize ions, making them more reactive, while nonpolar solvents may hinder nucleophilic reactions.
3.5. Nucleophilic Substitution and Addition
Nucleophiles are key players in nucleophilic substitution and addition reactions. In nucleophilic substitution, a nucleophile replaces a leaving group in a molecule. In nucleophilic addition, a nucleophile adds to an electrophilic center, often resulting in the formation of a new bond.
4. Key Differences
With a solid understanding of the properties of bases and nucleophiles, let’s now explore the key differences between these two essential chemical entities:
| Property | Bases | Nucleophiles |
|---|---|---|
| Role | Accept protons or donate lone pair electrons | Attack electrophiles to form new bonds |
| Electron Density | May or may not be electron-rich | Electron-rich |
| pH Increase | Raises pH by forming hydroxide ions | No direct impact on pH |
| Typical Examples | Hydroxide ions (OH-), ammonia (NH3) | Chloride ions (Cl-), cyanide ions (CN-), amines |
| Common Reactions | Acid-base reactions | Nucleophilic substitution and addition |
| Sensory Properties | Slippery feel and bitter taste (some) | No distinctive sensory properties |
| Strength | Can be strong or weak | Varies in nucleophilicity |
Now, let’s break down these differences in more detail:
4.1. Role
The fundamental difference between bases and nucleophiles lies in their roles in chemical reactions. Bases either accept protons (H+) or donate lone pair electrons, while nucleophiles primarily function by attacking electrophiles to form new chemical bonds.
4.2. Electron Density
Bases may or may not be electron-rich. They are characterized by their ability to accept or donate electrons. In contrast, nucleophiles are inherently electron-rich species, making them more inclined to share their electrons with electrophiles.
4.3. pH Increase
One of the practical effects of bases is their ability to raise the pH of a solution by generating hydroxide ions (OH-). Nucleophiles, on the other hand, do not directly impact the pH of a solution as their primary role is to participate in chemical reactions rather than alter the acidity or alkalinity of a medium.
4.4. Typical Examples
Common examples of bases include hydroxide ions (OH-), ammonia (NH3), and alkoxides (RO-). Nucleophiles, on the other hand, encompass a broader range of compounds, including chloride ions (Cl-), cyanide ions (CN-), and amines (RNH2).
4.5. Common Reactions
Bases are central to acid-base reactions, where they neutralize acidic protons. Nucleophiles, on the other hand, play pivotal roles in nucleophilic substitution and addition reactions, where they attack electrophilic centers in molecules.
4.6. Sensory Properties
Some bases, such as sodium hydroxide (NaOH), are known for their distinctive sensory properties, including a slippery feel and a bitter taste. In contrast, nucleophiles do not possess specific sensory characteristics that aid in their identification.
4.7. Strength
Bases can vary in strength, with some being strong (e.g., sodium hydroxide) and others being weak (e.g., ammonia). Nucleophiles, on the other hand, vary in nucleophilicity, which depends on factors such as electron density and steric hindrance. Nucleophilicity determines how reactive a nucleophile is in a given reaction.
5. Reactivity
Understanding the reactivity of bases and nucleophiles is essential for predicting and controlling chemical reactions. Here’s how these compounds behave in chemical reactions:
5.1. Reactivity of Bases
Bases are most reactive when they encounter acidic protons. In an acid-base reaction, a base accepts a proton, forming a new bond with the acidic species. Strong bases, such as sodium hydroxide (NaOH), are highly reactive and can completely deprotonate acidic compounds. Weaker bases, like ammonia (NH3), have limited reactivity and may only partially deprotonate acidic molecules.
5.2. Reactivity of Nucleophiles
Nucleophiles are highly reactive towards electrophiles. Electrophiles are species with electron-deficient centers, often characterized by a positive charge or partial positive charge. When a nucleophile encounters an electrophilic site in a molecule, it attacks and shares its electrons, leading to the formation of a new covalent bond. The reactivity of nucleophiles depends on their nucleophilicity, which varies from one nucleophile to another.
6. Applications
Both bases and nucleophiles find numerous applications in chemistry and various industries. Let’s explore some real-world examples of where these compounds come into play:
6.1. Applications of Bases
- Chemical Synthesis: Bases are used in the synthesis of various organic compounds. For instance, sodium hydroxide (NaOH) is employed in saponification reactions to produce soap.
- Pharmaceuticals: Bases are used in pharmaceutical manufacturing processes to synthesize drugs and pharmaceutical intermediates.
- Water Treatment: Bases like calcium hydroxide (Ca(OH)2) are used to treat water by neutralizing acidic impurities.
- Food Industry: Bases are used in food processing, such as the production of baked goods, where they interact with acidic ingredients like baking powder.
6.2. Applications of Nucleophiles
- Organic Synthesis: Nucleophiles play a crucial role in organic synthesis, enabling the creation of complex molecules. For example, Grignard reagents, which are strong nucleophiles, are used to form carbon-carbon bonds.
- Pharmaceuticals: Nucleophilic reactions are vital in pharmaceutical chemistry for the synthesis of drugs and the modification of existing compounds.
- Chemical Analysis: Nucleophiles are used in analytical chemistry techniques such as nucleophilic substitution reactions for identifying and quantifying specific functional groups in molecules.
- Environmental Remediation: Nucleophilic reactions can be employed to detoxify hazardous chemicals in the environment.
7. Additional Considerations
To further enhance your understanding of bases and nucleophiles, let’s delve into some additional considerations and practical insights:
7.1. Lewis Bases vs. Bronsted-Lowry Bases
While we have discussed bases in the context of Bronsted-Lowry theory, it’s important to note that there are different ways to define bases in chemistry. The Bronsted-Lowry theory focuses on proton transfer, where bases accept protons (H+ ions). However, the Lewis theory defines bases as electron pair donors. According to the Lewis definition, any species that can donate a pair of electrons qualifies as a base. This broader definition includes species that may not have the traditional characteristics of bases under the Bronsted-Lowry definition. For example, in the Lewis theory, even molecules like ammonia (NH3) can be considered bases because they can donate a lone pair of electrons.
7.2. Nucleophilicity and Leaving Groups
In nucleophilic substitution reactions, nucleophilicity plays a critical role. Nucleophilicity refers to the tendency of a nucleophile to participate in a nucleophilic reaction. It’s important to understand that the choice of leaving group in a molecule can influence nucleophilicity. A better leaving group will make the molecule more susceptible to nucleophilic attack. For instance, in the context of alkyl halides, iodine (I-) is a better leaving group compared to fluorine (F-) because it is more polarizable and can stabilize the negative charge that forms upon leaving.
7.3. Solvent Effects
The choice of solvent in a chemical reaction can significantly impact the behavior of both bases and nucleophiles. Polar solvents tend to enhance the reactivity of both bases and nucleophiles. This is because polar solvents stabilize ions and provide a suitable environment for ionic reactions to occur. Nonpolar solvents, on the other hand, may hinder the reactivity of these species.
7.4. Acid-Base Equilibria
In many chemical reactions, especially those involving bases, understanding acid-base equilibria is essential. The concept of pKa, which represents the negative logarithm of the acid dissociation constant (Ka), is valuable in predicting the behavior of bases. Bases with a pKa higher than the pH of the solution will readily accept protons, while those with a pKa lower than the pH will not.
7.5. Practical Synthesis
In laboratory settings, the choice of base or nucleophile can significantly impact the success of a chemical synthesis. Chemists carefully select these reagents based on the desired reaction and the specific functional groups they wish to manipulate. Additionally, factors such as temperature, concentration, and reaction time play vital roles in achieving the desired outcomes.
8. Future Developments and Applications
The understanding of bases and nucleophiles continues to evolve as new research and technologies emerge in the field of chemistry. Future developments may lead to the discovery of novel bases and nucleophiles with unique properties and reactivities. These advancements could open up new possibilities for chemical synthesis, drug development, and environmental remediation.
One exciting area of research is the design and synthesis of highly selective bases and nucleophiles. Chemists are working on developing reagents that can target specific functional groups in complex molecules, allowing for more precise and efficient chemical transformations.
Furthermore, the application of bases and nucleophiles in green chemistry and sustainable processes is gaining attention. Efforts are being made to reduce the environmental impact of chemical reactions by using milder bases and nucleophiles that generate less waste and have lower toxicity.
FAQs
In chemistry, a base is a substance that can accept a proton (H+) or donate a pair of electrons to form a new chemical bond. Bases play a central role in acid-base reactions and are known for raising the pH of a solution. On the other hand, a nucleophile is a species rich in electrons that seeks out positively charged nuclei to form new bonds. The key difference lies in their roles: bases primarily participate in acid-base reactions, while nucleophiles are involved in nucleophilic substitution and addition reactions by attacking electrophiles.
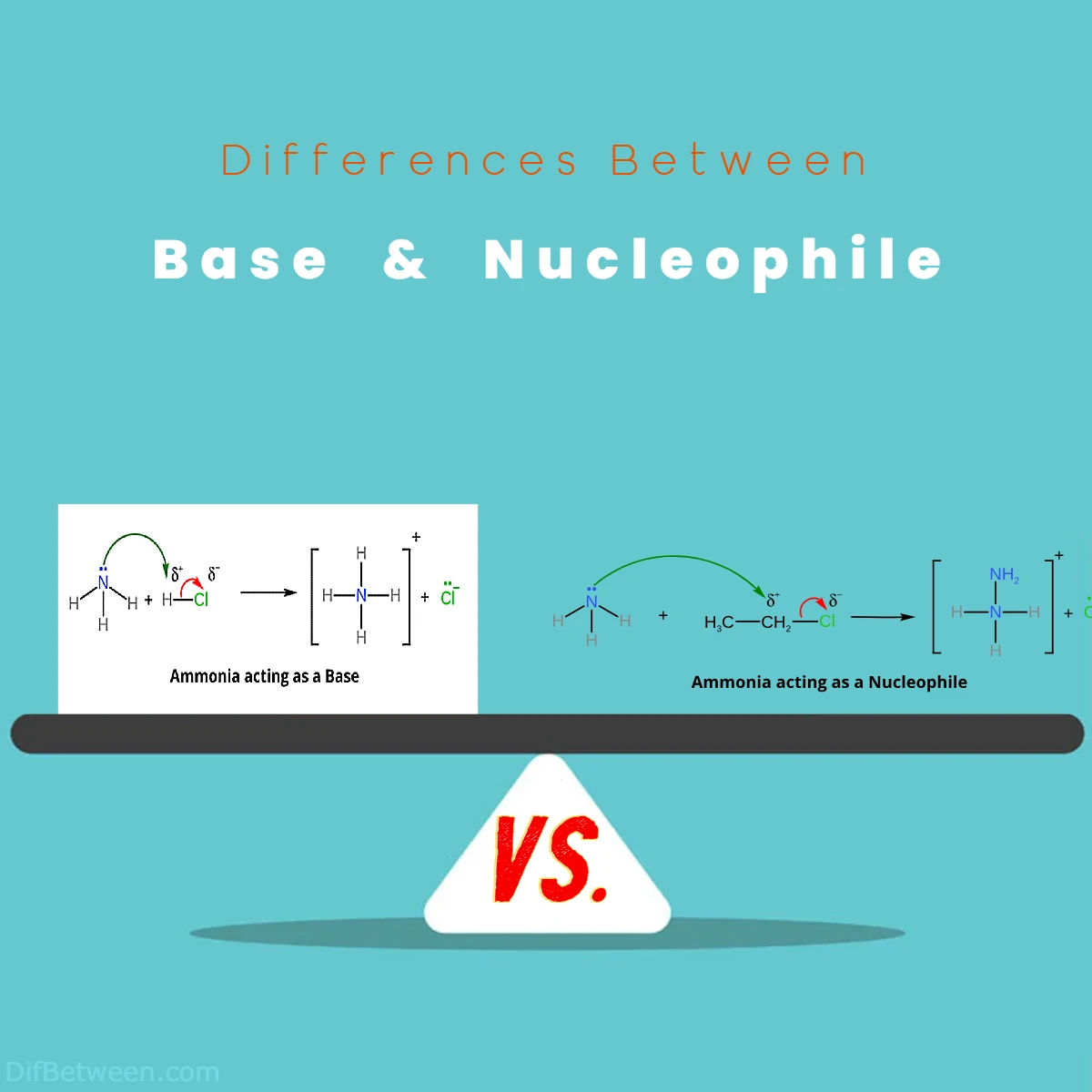
Yes, some compounds can exhibit dual behavior as both bases and nucleophiles, depending on the specific reaction conditions. For instance, ammonia (NH3) can act as a base by accepting a proton but can also behave as a nucleophile by donating a lone pair of electrons to form a new bond. The distinction often depends on the nature of the reactants and the reaction environment.
Bases have the ability to raise the pH of a solution by generating hydroxide ions (OH-), making it more alkaline. Nucleophiles, in contrast, do not directly impact pH levels as their primary function is not related to altering the acidity or alkalinity of a solution. Their role is focused on participating in chemical reactions rather than changing the pH.
Some common bases, such as sodium hydroxide (NaOH), may exhibit distinctive sensory properties. They can have a slippery feel and a bitter taste. However, nucleophiles typically do not possess specific sensory characteristics that aid in their identification. Identification of nucleophiles usually relies on their chemical behavior and properties.
Certainly! Bases find applications in various industries, including chemical synthesis, pharmaceuticals, water treatment, and the food industry. For instance, they are used in soap production, pharmaceutical manufacturing, and as water softeners. Nucleophiles are crucial in organic synthesis, pharmaceutical chemistry, chemical analysis, and environmental remediation. They are used in creating complex organic molecules, drug development, and detoxifying hazardous chemicals in the environment.
Nucleophilicity varies significantly among different nucleophiles. It depends on factors such as electron density, steric hindrance, and the nature of the nucleophile. Highly nucleophilic species are more reactive and have a greater tendency to participate in nucleophilic reactions, while less nucleophilic species are less reactive and may require specific reaction conditions to be effective. The choice of nucleophile is critical in designing chemical reactions for specific outcomes.
Read More:
Contents