| Aspect | Molecule | Atom |
|---|---|---|
| Definition | A group of atoms bonded together, forming a distinct entity | The fundamental unit of matter and chemical elements |
| Composition | Composed of two or more atoms of the same or different elements | Composed of subatomic particles – protons, neutrons, and electrons |
| Bonding | Formed through chemical bonds | No bonds; individual entities |
| Structure | Has a specific arrangement of atoms | Nucleus at the center with electrons orbiting around it |
| Behavior | Exhibits properties distinct from constituent atoms | Properties determined by atomic number and periodic table |
| Role in Matter | Constitutes compounds and substances | Forms elements and participates in chemical reactions |
| Properties | Characteristics influenced by arrangement and bonding | Characteristics based on atomic number and electron structure |
| Stability and Reactivity | Reactivity influenced by bonding and arrangement | Reactivity determined by electron configuration |
| Quantum Mechanics | Exhibits quantized vibrational and rotational energy levels | Governs behavior at subatomic scales |
| Environmental Impact | Impacts climate through greenhouse gases | Can participate in nuclear reactions and energy generation |
| Role in Nanotechnology | Forms building blocks for engineered materials | Manipulated to engineer new materials and devices |
| Role in Medicine | Participates in biological processes and drug interactions | Used in pharmaceuticals and medical treatments |
| Cosmic Contribution | Detected in space through spectroscopy | Participates in nuclear fusion in stars |
| Interconnectedness | Contributes to unity and diversity in the universe | Creates unity among elements in the periodic table |
| Future Exploration | Continues to reveal molecular structures and behaviors | Enables deeper understanding and technological advances |
| Philosophical Aspect | Reflects human curiosity, interconnectedness, and wonder | Inspires contemplation about our place in the cosmos |
Picture atoms as the elemental wonders, akin to the magical Lego bricks that construct everything around us. These minuscule marvels, consisting of protons, neutrons, and electrons, team up to create a symphony of matter. On the other hand, molecules take the stage as intricate structures formed by the marriage of two or more atoms. Think of them as the architects crafting the diversity of compounds that fill our lives with color and meaning.
Differences Between Molecule and Atom
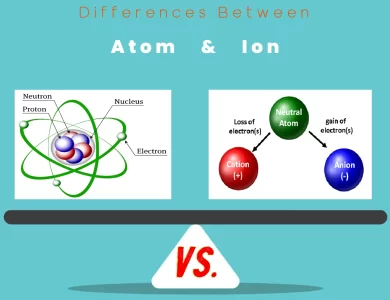
The main differences between molecules and atoms lie in their composition and behavior. Atoms are the fundamental units of matter, consisting of protons, neutrons, and electrons, whereas molecules are formed when atoms bond together, creating distinct entities with unique properties. Molecules exhibit characteristics that go beyond those of individual atoms, as their properties are determined by both their constituent atoms and the arrangement of their bonds. This differentiation underscores the crucial role that both molecules and atoms play in shaping the diversity and complexity of the world around us.
1. Essence of Existence: Molecules and Atoms Defined
Atoms: Picture a bustling marketplace filled with a myriad of colorful stalls – that’s akin to the bustling world of atoms. Atoms are the elemental units of matter, the indivisible particles that constitute all chemical elements. These submicroscopic entities are the true essence of existence, the primal entities that combine to craft the diverse array of matter present in our universe.
Molecules: Now, shift your perspective to a tranquil garden where petals interlace to form beautiful blooms – this mirrors the harmonious assembly of molecules. Molecules are composed of atoms that unite through chemical bonds, creating a cooperative entity with distinct properties. While atoms are the letters of the elemental alphabet, molecules are the words they form, each narrating a unique tale of substance and structure.
2. Building Blocks of Diversity: Composition and Arrangement
Atoms: Just like a painter uses a palette of colors to create a masterpiece, nature employs atoms to craft the elements. These atoms are composed of subatomic particles – protons, neutrons, and electrons. Protons, positively charged, and neutrons, neutrally charged, gather in the nucleus at the center, while the negatively charged electrons whirl around this nucleus, akin to planets encircling the sun.
Molecules: If atoms are the letters, molecules are the sentences composed using these letters. When atoms of different elements or the same element come together, they form molecules. The arrangement of these atoms defines the nature and behavior of the resulting molecule. Water, for instance, is a molecule formed by two hydrogen atoms bonded to a single oxygen atom, and this arrangement gives rise to its distinctive properties.
In essence, while atoms are the individual musical notes, molecules are the symphonies they create, and each molecular arrangement produces a unique harmony of characteristics.
3. Bonding Forces: The Glue of Molecular Cohesion
Atoms: Within the realm of atoms, the nucleus, bound by the strong nuclear force, keeps protons and neutrons closely knitted. Meanwhile, electrons, bearing negative charges, are held in orbit by the electromagnetic force, forming distinct energy levels or shells. These shells play a pivotal role in how atoms interact with each other.
Molecules: Imagine molecules as a gathering of friends, where friendships are forged through various connections. These connections, termed chemical bonds, are akin to the ties that bind atoms together within molecules. Covalent bonds involve atoms sharing electrons to achieve stability, while ionic bonds stem from the attractive forces between positively and negatively charged ions. Furthermore, hydrogen bonds, though weaker, play a significant role in molecular interactions, especially in substances like water and biological molecules.
4. Play of Properties: Unique Traits of Molecules and Atoms
Atoms: Just as each star in the night sky has its distinct luminosity, each atom boasts its own set of properties. These properties are determined by the atomic number, which corresponds to the number of protons. Elements are classified on the periodic table based on their atomic numbers, showcasing a periodic recurrence of properties.
Molecules: When atoms combine to form molecules, a fascinating transformation occurs. Molecules inherit characteristics not only from their constituent atoms but also from their arrangement and bonding. This is vividly evident in compounds like diamonds and graphite, both composed solely of carbon atoms, yet possessing vastly different properties due to their distinct molecular arrangements.
5. Dynamic Existence: Stability and Reactivity
Atoms: In the serene dance of the cosmos, atoms find stability by achieving a full outer electron shell. Elements strive to attain the electron configuration of noble gases, renowned for their stability. This drive dictates the reactivity of atoms – some elements readily relinquish electrons to become positively charged ions, while others eagerly capture electrons to form negative ions.
Molecules: Molecules, too, seek stability, often through sharing or exchanging electrons. The nature of the bonds within a molecule determines its stability and reactivity. Some molecules are inherently stable and unreactive under normal conditions, while others are poised to engage in chemical reactions, exchanging partners to achieve a more balanced arrangement.
6. Grandeur of Complexity: From Simple to Complex
Atoms: In the mosaic of the universe, atoms are the solitary tiles that pave the way for grander patterns. Yet, they do not remain solitary for long, often coming together to create molecules, which in turn build intricate structures.
Molecules: As molecules emerge from the embrace of atoms, complexity flourishes. From simple diatomic molecules like oxygen (O2) to the breathtaking complexity of DNA’s double helix, molecules are the architects of life’s diversity. These complex molecules hold the blueprints for biological processes, intricately dictating the dance of life itself.
7. Unified Diversity: Cooperation of Atoms in Molecules
Atoms: While atoms are the solitary wanderers, they find unity in their numbers. Elements, represented on the periodic table, symbolize the diverse array of atoms in existence. Each element brings its unique atomic number, mass, and properties to the tableau of nature.
Molecules: In molecules, atoms unite harmoniously, creating entirely new substances with properties that can differ drastically from the individual atoms. The cooperation of atoms through bonds forms the basis for the immense diversity of compounds, from the nourishing glucose fueling our cells to the awe-inspiring vastness of polymeric materials.
8. Impact on Matter: Tracing Influence in Chemistry
Atoms: The trail of atoms in the world of chemistry is akin to the footsteps of explorers in uncharted territories. The manipulation of individual atoms, a realm known as nanotechnology, holds the potential to revolutionize industries and technology, allowing us to engineer materials atom by atom for unprecedented performance.
Molecules: The dance of molecules, on the other hand, is the choreography of chemical reactions. The interactions and transformations of molecules steer the course of countless chemical processes, from the combustion that propels engines to the intricate cascade of reactions driving the synthesis of pharmaceuticals.
9. Exploring the Cosmos: Molecules and Atoms Beyond Earth
Atoms and Molecules Beyond Earth: The captivating dance of molecules and atoms extends far beyond the confines of our planet. In the cosmos, molecules play a vital role in unraveling the mysteries of the universe. Spectroscopy, a technique that examines the light emitted or absorbed by molecules, provides astronomers with insights into the composition, temperature, and even motion of celestial bodies. Molecules like water, carbon dioxide, and methane have been detected in the atmospheres of distant planets, shedding light on their potential habitability and geological processes.
Atoms in Cosmic Furnaces: Within the heart of stars, atoms engage in a cosmic ballet that generates the energy and elements essential for life. Nuclear fusion, the process by which atomic nuclei combine to release energy, takes place in the searing temperatures of stars. This process fuses lighter elements into heavier ones, producing elements like carbon, oxygen, and even the precious metals that dot the cosmos. When massive stars exhaust their nuclear fuel, they explode in dazzling supernovae, scattering these newly formed elements across the universe, ready to participate in the formation of new stars, planets, and, ultimately, life.
10. Quantum Mechanics: A Peek into the Subatomic Realm
Atoms and Quantum Mechanics: The realm of atoms and molecules delves into the mysterious world of quantum mechanics, a branch of physics that describes the behavior of particles at the smallest scales. At the quantum level, particles like electrons don’t have well-defined paths but exist as probability distributions, challenging our classical intuitions. Quantum mechanics governs the behavior of electrons around atomic nuclei, dictating the structure of atoms and their interactions with other atoms to form molecules.
Molecules as Quantum Entities: Molecules, too, exhibit quantum properties. Their vibrational and rotational energy levels are quantized, meaning they can only possess certain discrete energy values. This quantization influences the absorption and emission of light by molecules, enabling scientists to deduce molecular structures and dynamics through spectroscopic techniques. Quantum mechanics is the underlying framework that guides the behavior of both atoms and molecules, shaping the astonishing diversity and complexity we observe.
11. Crafting New Frontiers: Nanotechnology and Beyond
Atoms in Nanotechnology: As our understanding of atoms has deepened, scientists have embarked on a remarkable journey into the realm of nanotechnology. This frontier involves manipulating individual atoms and molecules to engineer new materials and devices with unprecedented properties. The ability to arrange atoms with precision allows for the creation of materials with tailored characteristics, ranging from ultra-strong and lightweight materials to highly efficient electronic components. Nanotechnology holds the promise of revolutionizing industries from electronics to medicine, shaping the future with atomic precision.
Molecules in Medicine: Beyond nanotechnology, molecules are at the heart of medicinal advancements. The field of pharmacology hinges on molecules’ interactions with biological systems. Medicinal chemists design molecules that selectively target specific cellular processes, offering treatments for diseases ranging from infections to cancer. The intricate dance of molecules within our bodies guides health and well-being, and the synthesis of novel molecules offers pathways to better therapies and improved quality of life.
12. Environmental Impact: Molecules, Atoms, and Sustainability
Atoms and Molecules in the Environment: The impact of atoms and molecules isn’t limited to laboratories; it resonates throughout our environment. Molecules like greenhouse gases play a crucial role in regulating Earth’s temperature by trapping heat in the atmosphere. However, human activities have led to an increase in the concentration of these molecules, contributing to global warming. Understanding the composition and behavior of molecules in the atmosphere is essential for devising strategies to mitigate climate change.
Atoms in Renewable Energy: The search for sustainable energy sources has brought atoms to the forefront of innovation. The harnessing of nuclear energy involves the controlled fission of atomic nuclei, releasing a tremendous amount of energy. While challenges like waste disposal and safety persist, nuclear power presents a low-carbon alternative to conventional fossil fuels. Additionally, advancements in solar cells, which rely on the movement of electrons between atoms in materials like silicon, offer a clean and renewable energy solution.
13. Limitless Curiosity: The Future of Exploration
Unveiling the Microcosmos: The exploration of molecules and atoms is an ongoing journey fueled by boundless curiosity. Advancements in technology, such as scanning tunneling microscopes and particle accelerators, enable scientists to peer deeper into the microcosmos, unraveling the mysteries of atomic and molecular structures. This pursuit not only deepens our understanding of the fundamental nature of matter but also unveils pathways for groundbreaking discoveries across fields.
From Fundamental Science to Applications: The study of molecules and atoms isn’t confined to theoretical exploration alone; it bridges the gap between fundamental science and real-world applications. Insights into the behavior of molecules in extreme conditions aid in the design of materials for space exploration and high-tech industries. Furthermore, the understanding of atomic and molecular interactions informs the development of novel materials, pharmaceuticals, and energy solutions that shape our modern lives.
14. The Human Connection: A Philosophical Reflection
Atoms and Molecules in Human Experience: In the symphony of molecules and atoms, an intriguing philosophical dimension emerges. These microscopic entities weave the tapestry of life, connecting all living beings through shared elemental constituents. From the air we breathe to the sustenance we consume, our very existence is intertwined with the dance of molecules and atoms. This interconnectedness invites contemplation about our place in the cosmos and the profound unity that underlies our diversity.
Atoms of Wonder: The exploration of molecules and atoms isn’t just a scientific endeavor; it’s a testament to human curiosity and ingenuity. It reflects our innate drive to unravel the mysteries of existence, to peer into the unseen and understand the universe’s inner workings. The story of molecules and atoms is a story of human exploration, discovery, and the unrelenting pursuit of knowledge that defines our species.
FAQs
The fundamental distinction lies in their composition and structure. An atom is the smallest unit of an element, consisting of a nucleus with protons and neutrons, surrounded by electrons. On the other hand, a molecule is composed of two or more atoms bonded together, forming a distinct entity with unique properties.
Atoms have specific properties determined by their atomic number and their position in the periodic table. In contrast, molecules exhibit properties that arise not only from the individual atoms they contain but also from their arrangement and the type of chemical bonds between them. This results in a wide range of characteristics that go beyond those of individual atoms.
Atoms are the building blocks of elements, and they combine to form molecules. Molecules, in turn, constitute compounds and substances. This cooperation of atoms through bonding is what gives rise to the incredible diversity of matter we observe in the world.
Atoms do not have bonds; they exist as individual entities. Molecules, however, are formed through chemical bonds, where atoms share or exchange electrons to achieve stability. These bonds include covalent bonds (shared electrons), ionic bonds (attraction between oppositely charged ions), and hydrogen bonds (intermolecular forces).
Molecules play a pivotal role in astronomy, as their spectral lines provide insights into celestial bodies’ composition and properties. Atoms, especially in the heart of stars, engage in nuclear fusion, producing energy and the elements essential for life. Their behavior helps astronomers understand stellar processes and the evolution of the universe.
The study of atoms has led to the field of nanotechnology, allowing scientists to manipulate individual atoms for engineering purposes. Molecules contribute to fields like medicine, where their interactions drive pharmaceutical advancements and therapeutic solutions. Additionally, both atoms and molecules influence materials science, renewable energy, and electronics.
The interconnectedness of molecules and atoms underscores the unity of all matter. This reflection inspires contemplation about our place in the cosmos, highlighting the intricate relationship between the microcosm and the macrocosm. The pursuit of understanding these fundamental entities speaks to our innate curiosity as explorers of both the tangible and the unseen.
Read More:
Contents
- Differences Between Molecule and Atom
- 1. Essence of Existence: Molecules and Atoms Defined
- 2. Building Blocks of Diversity: Composition and Arrangement
- 3. Bonding Forces: The Glue of Molecular Cohesion
- 4. Play of Properties: Unique Traits of Molecules and Atoms
- 5. Dynamic Existence: Stability and Reactivity
- 6. Grandeur of Complexity: From Simple to Complex
- 7. Unified Diversity: Cooperation of Atoms in Molecules
- 8. Impact on Matter: Tracing Influence in Chemistry
- 9. Exploring the Cosmos: Molecules and Atoms Beyond Earth
- 10. Quantum Mechanics: A Peek into the Subatomic Realm
- 11. Crafting New Frontiers: Nanotechnology and Beyond
- 12. Environmental Impact: Molecules, Atoms, and Sustainability
- 13. Limitless Curiosity: The Future of Exploration
- 14. The Human Connection: A Philosophical Reflection
- FAQs