Chemistry
Welcome to our captivating category page that delves into the intriguing realm of chemistry! Here, you’ll embark on a journey of discovery as we unravel the mysteries behind the differences in terms and other important elements in the field of chemistry. Whether you’re a curious student, an avid learner, or simply someone with an interest in the subject, this collection of content will broaden your understanding and deepen your appreciation for the wonders of chemistry.
-

Normal Air vs Nitrogen in Tires
When it comes to tire maintenance, choosing between normal air and nitrogen inflation can impact your vehicle's performance and safety. Understanding the key differences between these two options is crucial for making an informed decision. Composition: Normal air consists of a mix of gases, including 78% nitrogen and 21% oxygen, along with trace elements. In contrast, nitrogen inflation provides nearly pure nitrogen, typically around 95-99% purity. Pressure Stability: Nitrogen-filled tires offer more stable pressure over time. The larger nitrogen molecules are less likely to escape through the tire's rubber, reducing the need for frequent top-ups, especially when compared to the oxygen permeation in normal air. Moisture Content: Normal air contains water vapor, which can lead to moisture accumulation within the tire. Nitrogen, being dry, prevents internal corrosion and rusting of tire components. Temperature Effects: Nitrogen is less sensitive to temperature changes, ensuring consistent pressure regardless of weather conditions. In contrast, normal air experiences significant pressure fluctuations with temperature variations. Permeation Rate: Nitrogen molecules have a slower leak rate compared to oxygen, resulting in more prolonged periods between pressure checks and top-ups. Availability and Cost: Normal air is readily accessible and often free at gas stations. Nitrogen inflation may come at a cost due to specialized equipment and materials, making it less convenient for some. Environmental Impact: Nitrogen is environmentally friendly, lacking harmful emissions and greenhouse gases found in normal air. By weighing these factors, you can decide whether nitrogen or normal air best suits your tire inflation needs and driving preferences.
-

Titre vs Dilution
In the realm of laboratory science, precision and accuracy reign supreme, and two fundamental techniques, dilution and titration, stand as pillars of analytical prowess. These methods, though related to the handling of solutions, serve vastly different purposes and involve distinct processes. Dilution, like a gentle brushstroke on a canvas, is the art of reducing the concentration of a solution by introducing a solvent. It is used to tailor solutions for various reasons, including safety, precision, and cost-efficiency. The process relies on mathematical calculations, making it a straightforward and essential technique in fields such as pharmaceuticals, chemical analysis, and biological research. In contrast, titration is akin to a scientific detective's quest for answers. It is a meticulously controlled chemical reaction between an analyte of unknown concentration and a titrant of known concentration. The key to titration lies in the precise detection of the endpoint, often signaled by a dramatic change in color due to the use of indicators. This technique is paramount in analytical chemistry, enabling scientists to unravel the mysteries of unknown concentrations and ensure product quality control in the pharmaceutical industry.
-

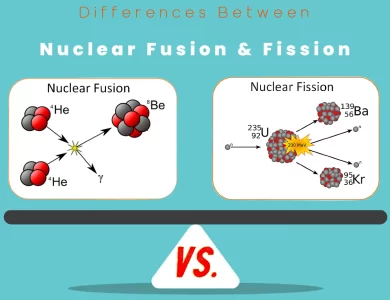
Fission vs Nuclear Fusion
Nuclear energy, with its vast potential and diverse applications, is a topic of immense significance in the quest for clean and sustainable power sources. Two distinct nuclear processes, fusion, and fission, stand at the forefront of this scientific exploration. Understanding the fundamental disparities between these processes is pivotal in unraveling their unique attributes and contributions to the energy landscape. Nuclear fusion, often hailed as the "holy grail" of clean energy, involves the fusion of light atomic nuclei, typically hydrogen isotopes, to form a heavier nucleus. This fusion process mirrors the energy generation mechanism of stars, releasing an extraordinary amount of energy. In contrast, nuclear fission revolves around the splitting of heavy atomic nuclei, like uranium or plutonium, into smaller nuclei, accompanied by the release of substantial energy. Both processes are awe-inspiring in their own right, but they diverge significantly in terms of fuel, energy release, waste generation, and safety. Fusion is characterized by its minimal waste production, low radioactivity, and an almost boundless fuel supply derived from hydrogen isotopes, making it an attractive prospect for a sustainable energy future. Moreover, fusion carries no risk of a nuclear meltdown, enhancing its safety profile. However, it demands the creation and maintenance of extreme conditions, including temperatures in the millions of degrees, which remain a formidable technical challenge. In contrast, fission reactors, which are already operational worldwide, provide a substantial portion of our current energy needs. Yet, they generate significant amounts of radioactive waste, pose safety concerns, and rely on limited fissile materials.
-

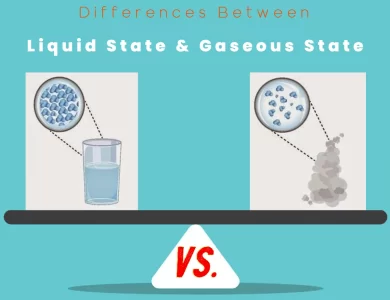
Gaseous State vs Liquid State
Join us on a captivating journey as we unravel the intriguing distinctions between the liquid state and the gaseous state of matter. In the realm of science, these two phases exhibit unique behaviors and properties that play a significant role in our daily lives and numerous industries. At the molecular level, liquids are characterized by closely packed molecules that retain a definite volume while conforming to the shape of their container. Their fluidity and viscosity allow for easy pouring, making them essential in various applications, from quenching your thirst with a refreshing beverage to facilitating chemical reactions in laboratories. On the other hand, gases present a vastly different molecular arrangement, with molecules far apart and exhibiting high kinetic energy. They possess neither a definite volume nor shape and have remarkable compressibility. Gases are the driving force behind numerous industrial processes, energy production, and even the air we breathe. Understanding these disparities is crucial, whether you're a scientist delving into the mysteries of matter or simply curious about the world around you. Join us as we delve into the fascinating "Differences Between Liquid State vs. Gaseous State" and uncover their significance in our everyday existence.
-

Fast HPLC vs HPLC
When it comes to liquid chromatography, two powerful techniques stand out: High-Performance Liquid Chromatography (HPLC) and its rapid sibling, Fast HPLC (High-Speed or Ultra-Fast Liquid Chromatography). These analytical methods share a common goal—separating and quantifying compounds within complex mixtures—but they diverge significantly in their approach. HPLC, characterized by its meticulous precision and methodical pace, excels in delivering high-resolution separations. It's the choice for applications where every detail matters, from pharmaceutical quality control to in-depth research involving intricate sample matrices. In contrast, Fast HPLC, as its name suggests, prioritizes speed without sacrificing resolution to the same degree. This technique is the go-to option for high-throughput laboratories where efficiency and rapid results are paramount. Delving into the nuances reveals that HPLC operates at moderate pressures, typically around 6,000 to 7,000 psi, while Fast HPLC boldly ventures into the high-pressure realm, often exceeding 10,000 psi. This higher pressure environment in Fast HPLC is the trade-off for its remarkable speed, demanding robust instrumentation capable of handling the stress. Sample size handling also differs, with HPLC offering versatility across a wide range of volumes and Fast HPLC optimizing for smaller to medium-sized sample volumes.
-

GC vs HPLC
In the realm of analytical chemistry, the choice between High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC) can be as critical as choosing the right tool for a complex task. Both HPLC and GC are indispensable techniques used to separate, identify, and quantify compounds in a variety of samples, but they employ different principles and are suited for distinct applications. HPLC, as the name suggests, employs a liquid mobile phase, making it highly versatile. It can handle a wide range of sample types, from liquids to solids, and is renowned for its compatibility with non-volatile compounds. This makes HPLC a valuable tool in industries like pharmaceuticals, food and beverage, clinical diagnostics, and environmental monitoring. While it offers moderate sensitivity, HPLC's ability to handle diverse samples and its robustness make it a popular choice for routine analytical tasks. On the other side of the spectrum, we have GC, a technique that relies on a vaporized gas mobile phase. This characteristic makes GC exceptionally well-suited for the analysis of volatile compounds. It's celebrated for its high sensitivity, capable of detecting trace-level compounds with precision. GC finds its niche in applications such as environmental monitoring, where minute concentrations of pollutants need to be identified, and in forensic science, where the detection of volatile substances plays a crucial role.
-

UPLC vs HPLC
When it comes to chromatography, precision is paramount. High-Performance Liquid Chromatography (HPLC) and Ultra-Performance Liquid Chromatography (UPLC) are two prominent techniques in the world of analytical chemistry. In our detailed analysis, we uncover the primary distinctions between these chromatographic methods. From particle sizes and operating pressures to resolution, sensitivity, and cost considerations, we break down the factors that can influence your choice between HPLC and UPLC. HPLC, with its versatile and cost-effective approach, remains a steadfast choice for various applications. On the other hand, UPLC's cutting-edge technology offers exceptional speed, resolution, and sensitivity, making it the preferred option for demanding analyses. Whether you prioritize reliability, versatility, and affordability or require blazing speed and precision, our exploration of HPLC vs UPLC will guide you toward the chromatographic technique best suited to your laboratory's objectives.
-

Bromocresol Purple vs Bromocresol Blue
In the realm of chemistry and laboratory experiments, nuances matter. Enter Bromocresol Blue and Bromocresol Purple, two pH indicators that might look similar but play very different roles. Bromocresol Blue, with its vibrant blue to yellow shift, thrives in the pH range of 6.0 to 7.6, making it a go-to for detecting slight pH changes. On the other hand, Bromocresol Purple, transitioning from yellow to purple in the pH range of 5.2 to 6.8, is your choice for pinpoint accuracy in mildly acidic to mildly alkaline solutions. Dive into the colorful world of these indicators, understand their unique properties, and choose the right one for your experiments. Whether you're a seasoned scientist or an aspiring chemist, this comparison will help you make informed decisions and uncover the secrets of pH monitoring in the laboratory.
-

Divergent Evolution vs Convergent
In the grand tapestry of life on Earth, the forces of evolution have woven a complex and mesmerizing narrative. At its core, this narrative is shaped by two distinct yet intertwined processes: convergent and divergent evolution. While these terms might sound like scientific jargon, they hold the keys to understanding how species adapt and diversify over time. Convergent Evolution: Imagine two species, separated by oceans and eons of evolution, developing remarkably similar traits independently. Convergent evolution is the fascinating phenomenon where unrelated organisms evolve analogous features due to shared environmental challenges. Picture the wings of a bat and a bird – although their lineages couldn't be more different, both have evolved wings to achieve the same purpose: flight. Divergent Evolution: On the flip side, we have divergent evolution. It's the story of a common ancestor giving rise to multiple descendants, each marching to the beat of its ecological niche. Over time, these descendants accumulate differences, ultimately becoming unique species. Think of Darwin's finches, whose beak shapes adapted to various diets on the Galápagos Islands.
-

Food Web vs Food Chain
In the intricate web of life on Earth, there's an underlying system that dictates who eats whom. It's a fundamental concept in ecology, and it helps us understand how energy and nutrients flow through ecosystems. In this journey through the natural world, we'll delve into the key differences between food chains and food webs, shedding light on their unique characteristics and importance. Food Chains: Food chains are like the basic building blocks of ecological relationships. They represent a simplified, linear flow of energy and nutrients from one organism to another, highlighting who eats whom in a straightforward manner. Think of them as the "who eats who" diagrams of the natural world. Food Webs: Food webs, on the other hand, are like the intricate tapestries of the natural world. While food chains are linear and simplistic, food webs depict the complex network of interconnected food chains within an ecosystem. In essence, a food web is a more accurate representation of the real-world relationships among organisms.