| Property | Ethane | Ethanol |
|---|---|---|
| Chemical Formula | C2H6 | C2H5OH |
| Chemical Structure | Linear hydrocarbon | Alcohol compound with hydroxyl group |
| State at 25°C | Gas | Liquid |
| Odor | Odorless | Characteristic odor |
| Boiling Point | -128.2°C (-198.8°F) | 78.37°C (173.1°F) |
| Melting Point | -183.3°C (-297.9°F) | -114.1°C (-173.4°F) |
| Miscibility in Water | Negligible | Miscible |
| Source | Natural gas | Fermentation, synthesis |
| Primary Use | Fuel, petrochemical feedstock | Alcoholic beverages, industry |
| Applications | Limited | Wide-ranging |
| Reactivity | Limited | Versatile |
| Energy Content | High | Lower |
| Environmental Impact | Combustion emissions | Renewable biofuel, reduced emissions |
| Molecular Polarity | Non-polar | Polar |
| Toxicity | Low | Variable |
| Flammability | Highly flammable | Flammable |
| Chemical Synthesis | Limited pathways | Multiple routes |
| Economic Significance | Petrochemical industry | Multifaceted economy |
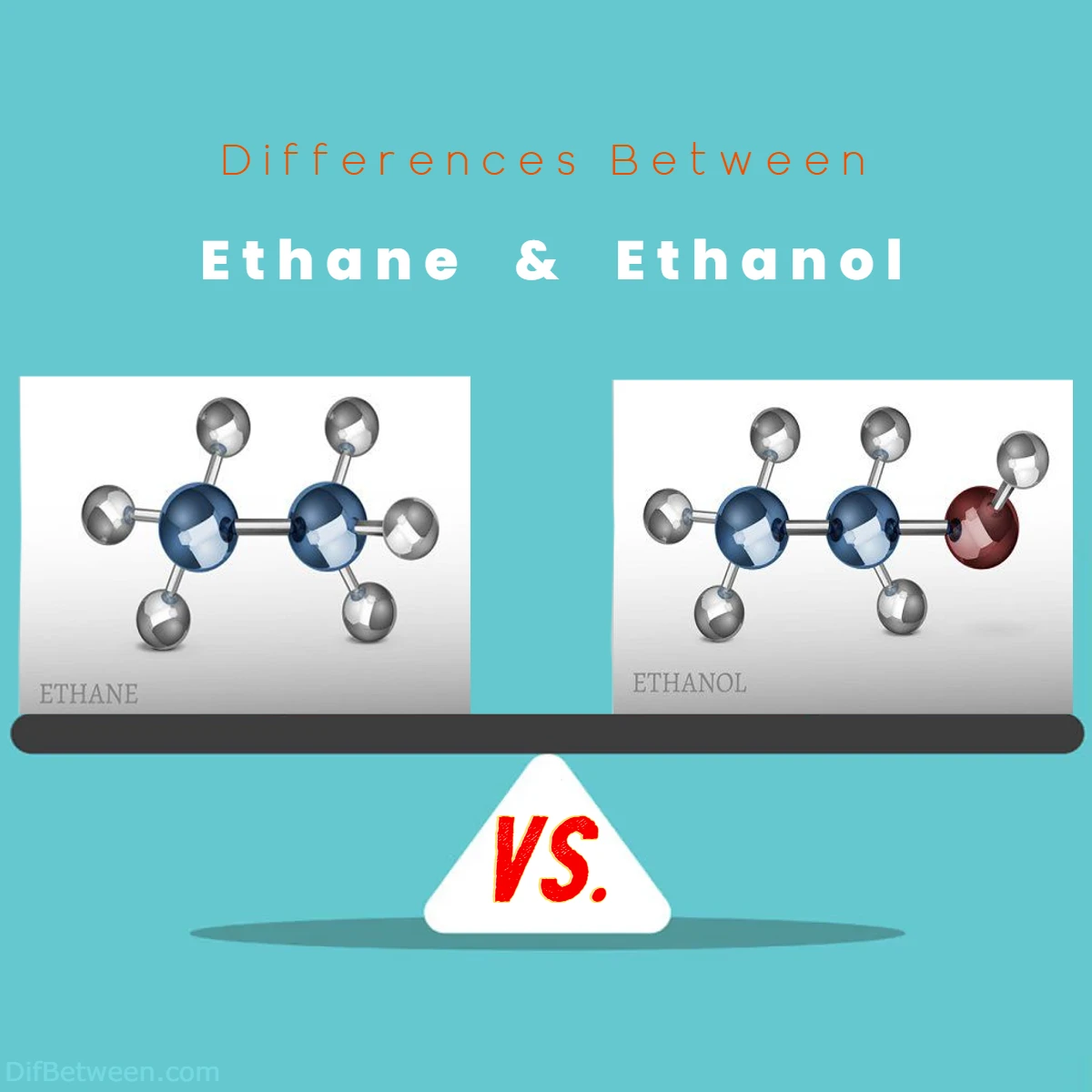
Ethane, the unsung hero of the petrochemical realm, takes center stage with its linear hydrocarbon structure. Imagine a string of carbon and hydrogen atoms dancing together in perfect harmony, creating a molecule that resides in the gaseous state. On the other hand, meet ethanol, the life of the party in the molecular world. With its characteristic odor and liquid form, ethanol introduces us to the realm of alcohols, where a hydroxyl group adds a touch of polarity and versatility.
Differences Between Ethane and Ethanol
The main differences between Ethane and Ethanol lie in their chemical compositions, physical properties, and applications. Ethane, represented by the formula C2H6, is a simple hydrocarbon gas with no odor, while Ethanol, with the formula C2H5OH, is a liquid alcohol known for its distinct smell. Ethane is primarily used as a fuel and petrochemical feedstock, whereas Ethanol finds application in alcoholic beverages, industries, and even as a renewable biofuel. These compounds differ in terms of boiling and melting points, reactivity, toxicity, and economic significance, making them stand out in distinct niches within the realm of chemistry and industry.
1. Chemical Structure and Composition
Ethane: The Simple Hydrocarbon
Ethane is a hydrocarbon, which means it consists solely of hydrogen and carbon atoms. Its chemical formula is C2H6, indicating that it comprises two carbon atoms and six hydrogen atoms. Structurally, ethane is a straightforward molecule, with the carbon atoms bonded in a single covalent bond. This simple structure forms a linear molecule, resembling a chain of atoms.
Ethanol: The Remarkable Alcohol
Ethanol, on the other hand, is an alcohol compound, denoted by the chemical formula C2H5OH. It consists of two carbon atoms, six hydrogen atoms, and one oxygen atom. The distinctive feature of ethanol is its hydroxyl (-OH) functional group, which is responsible for its classification as an alcohol. This functional group also accounts for ethanol’s unique properties and reactivity.
2. Physical Properties
Ethane: Gaseous and Odorless
Ethane manifests as a colorless, odorless, and tasteless gas at room temperature and standard atmospheric pressure. It has a boiling point of approximately -128.2°C (-198.8°F) and a melting point of around -183.3°C (-297.9°F). Ethane is notably lighter than air, which causes it to rise when released into the atmosphere.
Ethanol: Liquid with Characteristic Odor
In contrast, ethanol is a clear, colorless liquid at room temperature, emitting a distinct and recognizable odor. It has a significantly higher boiling point of approximately 78.37°C (173.1°F) and a melting point of around -114.1°C (-173.4°F). Ethanol is also miscible with water in all proportions, making it a valuable solvent in various industries.
| Property | Ethane | Ethanol |
|---|---|---|
| State at 25°C | Gas | Liquid |
| Odor | Odorless | Characteristic odor |
| Boiling Point | -128.2°C (-198.8°F) | 78.37°C (173.1°F) |
| Melting Point | -183.3°C (-297.9°F) | -114.1°C (-173.4°F) |
| Miscibility | Negligible in water | Miscible in water |
3. Sources and Production
Ethane: Abundant in Natural Gas
Ethane is primarily obtained as a component of natural gas. It’s separated from other hydrocarbons through processes like fractional distillation. It’s commonly used as a fuel and as a feedstock in the production of ethylene, a crucial compound in the plastics industry.
Ethanol: Fermentation and Synthesis
Ethanol is obtained through fermentation and chemical synthesis. It’s produced by the fermentation of sugars by yeast or bacteria. This process is employed in the production of alcoholic beverages. Additionally, ethanol is synthesized through the hydration of ethylene in the presence of a catalyst. This method yields industrial ethanol used in various applications, including as a fuel additive.
4. Applications and Uses
Ethane: Fuel and Feedstock
Ethane’s primary use is as a fuel. It’s burned to produce heat, light, and energy. In industrial settings, it serves as a feedstock for the production of ethylene and other chemicals, which are essential in the manufacture of plastics, rubber, and various chemical products. Ethane’s role in the petrochemical industry is pivotal, contributing to the creation of numerous everyday items.
Ethanol: Diverse Applications
Ethanol boasts a wide range of applications. In the beverage industry, it’s a key ingredient in alcoholic drinks. However, ethanol’s uses extend far beyond this realm. It’s employed as a solvent in industries such as pharmaceuticals and cosmetics. Ethanol is also blended with gasoline as a biofuel additive, contributing to reduced vehicle emissions. Furthermore, it serves as an antiseptic and disinfectant in healthcare.
| Application | Ethane | Ethanol |
|---|---|---|
| Fuel | Yes | Yes |
| Feedstock | Yes | No |
| Alcoholic beverages | No | Yes |
| Solvent | No | Yes |
| Biofuel additive | No | Yes |
| Pharmaceutical uses | No | Yes |
| Cosmetics | No | Yes |
| Antiseptics | No | Yes |
5. Environmental Impact
Ethane: Combustion Emissions
When ethane is burned as a fuel, it primarily produces carbon dioxide (CO2) and water vapor. While it’s a cleaner-burning fuel compared to coal or oil, it still contributes to greenhouse gas emissions. Efforts to reduce its environmental impact include implementing more efficient combustion technologies and capturing carbon emissions.
Ethanol: Biofuel Advantages
Ethanol is often considered a more environmentally friendly alternative to fossil fuels. It’s a renewable resource, as it’s derived from crops containing sugars or starches. When burned, ethanol releases carbon dioxide, but the carbon dioxide released during combustion is roughly equivalent to what the plant absorbed during its growth. This cycle helps mitigate the overall increase in atmospheric carbon dioxide.
6. Safety Considerations
Ethane: Inhalation Hazard
Ethane, being a highly flammable gas, poses a fire and explosion hazard in the presence of an ignition source. Inhalation of high concentrations of ethane can lead to oxygen deprivation, as it can displace air in confined spaces.
Ethanol: Flammability and Toxicity
Ethanol is flammable, and its vapors can form explosive mixtures in the air. Ingestion of ethanol in large quantities can result in alcohol poisoning. Prolonged exposure to ethanol vapor may also lead to health issues, including respiratory and central nervous system effects.
7. Chemical Reactivity
Ethane: Limited Reactivity
Due to its simple structure, ethane exhibits limited reactivity. It undergoes combustion reactions with oxygen to produce carbon dioxide and water. However, ethane’s lack of functional groups limits its involvement in various chemical reactions compared to compounds with more complex structures.
Ethanol: Versatile Reactivity
Ethanol’s hydroxyl (-OH) functional group imparts significant reactivity to the molecule. This functional group allows ethanol to participate in various reactions, including oxidation, esterification, and dehydration. Ethanol can be oxidized to form acetic acid and other compounds, making it a vital precursor in the synthesis of numerous chemicals.
8. Energy Content
Ethane: High Energy Density
Ethane is valued for its high energy density as a fuel. When burned, it releases a substantial amount of heat energy. This makes it an efficient choice for applications where a concentrated energy source is required, such as in industrial furnaces or power generation.
Ethanol: Lower Energy Density
Ethanol’s energy density is lower compared to that of hydrocarbons like ethane. When used as a fuel, ethanol provides less energy per unit volume. However, ethanol’s renewable nature and potential to reduce greenhouse gas emissions make it an attractive alternative in certain applications, particularly as a biofuel.
| Property | Ethane | Ethanol |
|---|---|---|
| Energy Density | High | Lower |
| Combustion Heat | High | Lower |
| Renewability | Non-renewable | Renewable |
9. Molecular Polarity
Ethane: Non-Polar Molecule
Ethane’s symmetrical linear structure results in an overall non-polar molecule. This means that there is an equal distribution of charge across the molecule, leading to minimal interactions with polar solvents and molecules.
Ethanol: Polar Molecule
The presence of the hydroxyl (-OH) functional group in ethanol imparts polarity to the molecule. The oxygen atom is more electronegative than the hydrogen atoms, leading to an uneven distribution of charge. Ethanol can form hydrogen bonds with other polar molecules, contributing to its solubility in water and its ability to dissolve various compounds.
10. Toxicity and Effects
Ethane: Low Toxicity
Ethane is generally considered to be of low toxicity. It does not have significant harmful effects on human health under normal conditions of use. However, as with any flammable gas, there is a risk of fire and explosion in confined spaces.
Ethanol: Variable Effects
Ethanol’s effects on human health vary depending on the dosage and frequency of exposure. In moderate amounts, it is commonly consumed in alcoholic beverages. However, excessive consumption can lead to alcohol poisoning, liver damage, and other health issues. Ethanol’s flammable nature also presents fire and safety risks.
| Property | Ethane | Ethanol |
|---|---|---|
| Inhalation Hazard | Fire and explosion risk | Flammable vapors; health effects |
| Ingestion Effects | Low toxicity | Health effects at excessive consumption |
| Flammability | Highly flammable | Flammable |
11. Chemical Synthesis
Ethane: Limited Synthetic Pathways
Ethane’s simple structure limits its direct synthetic applications. It is primarily obtained from natural gas deposits and is often used as a starting material for the production of more complex compounds like ethylene.
Ethanol: Diverse Synthetic Routes
Ethanol’s versatility extends to its synthesis. Apart from fermentation, which is a biological process, ethanol can be synthesized through chemical processes like the hydration of ethylene. This flexibility in synthesis contributes to ethanol’s availability for various industrial applications.
12. Economic Significance
Ethane: Petrochemical Backbone
Ethane plays a pivotal role in the petrochemical industry as a feedstock for the production of ethylene, which is used to manufacture plastics, synthetic rubber, and other essential products. Its importance in this industry contributes significantly to its economic value.
Ethanol: Multifaceted Economy
Ethanol’s economic significance spans multiple sectors. It contributes to the economies of industries such as agriculture (as a biofuel feedstock), beverages, pharmaceuticals, cosmetics, and more. Ethanol’s diverse applications contribute to its economic relevance on a global scale.
FAQs
The fundamental difference lies in their chemical structures and properties. Ethane (C2H6) is a linear hydrocarbon gas, odorless and used as a fuel and feedstock in the petrochemical industry. Ethanol (C2H5OH), an alcohol, is a liquid with a distinct odor, utilized in various applications, including alcoholic beverages, pharmaceuticals, and even as a biofuel additive.
Ethane exists as a colorless, odorless gas at room temperature, while ethanol is a clear, liquid substance with a characteristic smell. Ethane has much lower boiling and melting points compared to ethanol, which has a higher boiling point due to hydrogen bonding in its structure.
Ethane’s main use is as a fuel and feedstock in the petrochemical industry for the production of plastics and chemicals like ethylene. Ethanol has diverse applications, from alcoholic beverages and pharmaceuticals to cosmetics and as a renewable biofuel.
Indeed, they are. Ethane’s simple structure limits its reactivity, mainly participating in combustion reactions. Ethanol, due to its hydroxyl group, is versatile in reactions like oxidation, esterification, and dehydration.
Ethanol is considered more environmentally friendly as it’s derived from renewable sources like plants and has the potential to reduce greenhouse gas emissions, whereas ethane’s combustion contributes to carbon dioxide emissions.
Ethane, being a flammable gas, poses fire and inhalation hazards. Ethanol, while flammable as well, has additional toxicity concerns, especially with excessive consumption.
Ethane’s economic significance lies in its role as a petrochemical feedstock. Ethanol, on the other hand, contributes to a multifaceted economy, spanning industries from agriculture to healthcare and fuel.
Certainly! Ethane is a non-polar, odorless hydrocarbon gas used as fuel and feedstock, while ethanol is a polar, odorous alcohol with applications in beverages, industries, and more.
Ethane has a higher energy density compared to ethanol, making it a more efficient fuel source when burned.
While ethanol can be used as a biofuel additive to reduce gasoline consumption and emissions, it may not replace gasoline entirely due to differences in energy content and infrastructure limitations.
Ethane is primarily obtained from natural gas deposits through processes like fractional distillation. Ethanol can be produced through fermentation of sugars by yeast or bacteria, or chemically via processes like ethylene hydration.
Ethanol is commonly used as an antiseptic due to its disinfecting properties, making it a staple in healthcare settings.
Read More: