| Characteristic | Ethanol | Methanol |
|---|---|---|
| Chemical Formula | C₂H₅OH | CH₃OH |
| Molecular Structure | Two carbon atoms (C₂), five hydrogen atoms (H₅), and one oxygen atom (O) | One carbon atom (C), three hydrogen atoms (H₃), and one oxygen atom (O) |
| Common Names | Ethyl alcohol, grain alcohol | Methyl alcohol, wood alcohol |
| Sources of Production | Fermentation of plant materials (e.g., grains, sugarcane) or petrochemical processes | Steam reforming of natural gas or historically from wood distillation |
| Applications | Alcoholic beverages, biofuel additive, solvent, antiseptic | Chemical synthesis, formaldehyde production, industrial fuel, fuel cells |
| Physical Properties | Lower melting point (-114.1°C), lower boiling point (78.37°C), density of 0.789 g/cm³, refractive index of 1.361 | Higher melting point (-97.6°C), lower boiling point (64.7°C), density of 0.791 g/cm³, refractive index of 1.329 |
| Health and Safety | Generally safe for consumption, but excessive consumption can lead to health issues | Highly toxic if ingested, causes severe metabolic acidosis and blindness |
| Energy Content | Lower energy content than gasoline, used as a biofuel additive | Lower energy content than gasoline and ethanol, used in racing cars and industrial processes |
| Use as Antifreeze | Used in engine antifreeze formulations | Historically used as antifreeze, but less common due to toxicity |
| Environmental Impact | Considered a renewable biofuel, lower carbon emissions compared to fossil fuels | Cleaner combustion compared to gasoline, production impact depends on feedstock |
| Economic Significance | Contributes to biofuel industry, agricultural stimulation | Important in chemical and industrial sectors, reliant on natural gas availability |
| Regulatory Considerations | Regulated for consumption and as a biofuel, varies by region | Strict regulations due to toxicity, denaturants added to prevent consumption |
| Global Production | Major producers include the United States and Brazil | Largest producer is China, significant production in Europe and Asia |
| Research Focus | Biofuel production methods, advanced fermentation, cellulosic ethanol | Efficient production methods, cleaner combustion technologies, carbon capture |
| Combustion Products | Carbon dioxide (CO₂) and water vapor | Carbon dioxide (CO₂) and formaldehyde |
| Energy Efficiency | Lower energy content than gasoline, slightly reduced fuel efficiency | Lower energy content than gasoline and ethanol, reduced mileage |
| Uses in Industry | Solvent, personal care products, pharmaceuticals | Chemical synthesis, plastics, textiles, adhesives |
| Melting Point | -114.1°C | -97.6°C |
| Boiling Point | 78.37°C | 64.7°C |
| Density | 0.789 g/cm³ | 0.791 g/cm³ |
| Refractive Index | 1.361 | 1.329 |
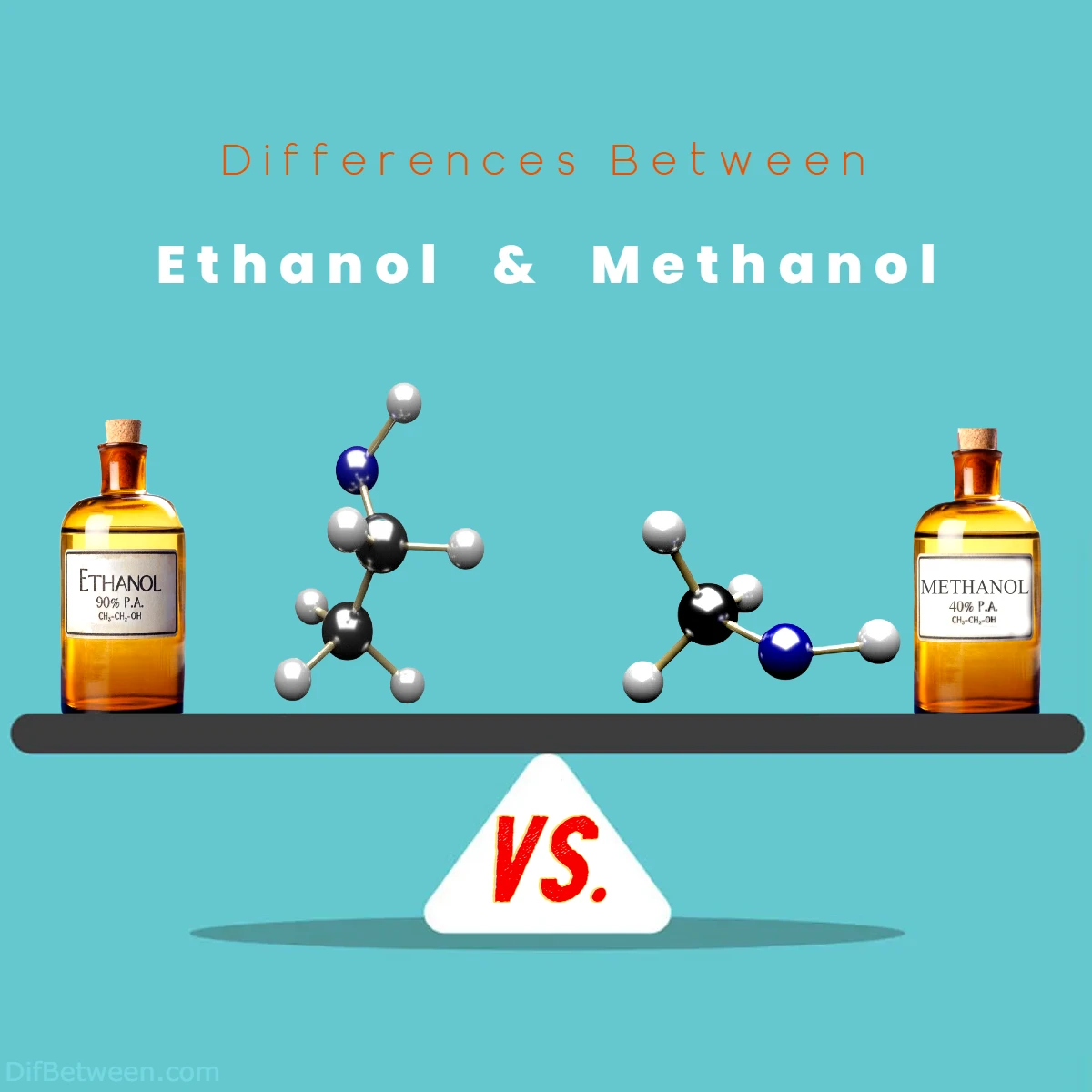
From the very moment these molecules come into being, their differences emerge. Ethanol, often known as ethyl alcohol or grain alcohol, boasts a molecular arrangement composed of two carbon atoms, five hydrogen atoms, and one oxygen atom. On the other hand, methanol, fondly dubbed wood alcohol or methyl alcohol, is characterized by its single carbon atom, three hydrogen atoms, and one oxygen atom. But the disparities don’t stop there! The sources from which these alcohols originate are as varied as their compositions.
Differences Between Ethanol and Methanol
The main differences between Ethanol and Methanol lie in their chemical structures, applications, and safety profiles. Ethanol, also known as ethyl alcohol, is composed of two carbon atoms, five hydrogen atoms, and one oxygen atom. It finds its place in alcoholic beverages, biofuel additives, and industrial solvents. In contrast, Methanol, or methyl alcohol, features a single carbon atom, three hydrogen atoms, and one oxygen atom. It is utilized primarily in chemical synthesis, formaldehyde production, and industrial processes. Additionally, Methanol’s toxic nature sets it apart, as excessive consumption can lead to severe health issues, making it unfit for consumption.
1. Chemical Structure and Composition
Ethanol: Ethanol, often referred to as ethyl alcohol or grain alcohol, boasts a chemical formula of C₂H₅OH. Its molecular structure features a chain of two carbon atoms (C₂), five hydrogen atoms (H₅), and an oxygen atom (O) bound together. This simple structure allows ethanol to partake in a variety of chemical reactions and interactions, making it a versatile compound with numerous applications.
Methanol: In contrast, methanol, known by names like wood alcohol or methyl alcohol, bears the chemical formula CH₃OH. Its molecular arrangement consists of a single carbon atom (C) bonded to three hydrogen atoms (H₃) and an oxygen atom (O). This uncomplicated structure grants methanol its own set of chemical properties and uses.
2. Sources and Production
Ethanol: Ethanol can be obtained through a natural fermentation process involving sugars and starches found in various plant materials. Common sources of ethanol production include grains like corn, barley, and wheat, as well as sugarcane and even fruits. The fermentation process is facilitated by yeast, which converts sugars into ethanol and carbon dioxide under anaerobic conditions. Additionally, ethanol can be synthesized through petrochemical processes, although this method is less common due to its higher cost.
Methanol: Methanol has diverse sources as well. Historically, it was primarily derived from the destructive distillation of wood, earning it the moniker “wood alcohol.” However, modern methanol production predominantly relies on a catalytic process called steam reforming. This method involves converting natural gas, rich in methane, into synthesis gas (syngas), which is a mixture of hydrogen and carbon monoxide. Syngas is then transformed into methanol through catalytic reactions.
3. Applications and Uses
Ethanol: Ethanol’s array of applications spans several domains. One of its most well-known uses is as an alcoholic beverage for consumption, often produced through the fermentation of various crops. Beyond the bar, ethanol plays a pivotal role as a biofuel additive, particularly in gasoline. This not only enhances octane ratings but also reduces emissions and dependence on fossil fuels. Ethanol’s solvent properties find application in industries such as pharmaceuticals, cosmetics, and personal care products. Additionally, ethanol serves as an antiseptic and disinfectant due to its ability to denature proteins, making it a staple in healthcare and sanitation.
Methanol: Methanol, with its lower cost and cleaner combustion, serves as a vital component in the production of formaldehyde, acetic acid, and a range of other chemicals. It’s a significant player in the creation of formaldehyde-based resins, which are integral to manufacturing wood products and textiles. Methanol’s combustibility also places it on the map as an industrial fuel and a potential energy source for fuel cells. However, it’s important to note that methanol’s toxic nature poses challenges in its application. Unlike ethanol, methanol is not suitable for consumption and can have severe health implications if ingested.
4. Physical Properties
| Property | Ethanol | Methanol |
|---|---|---|
| Melting Point | -114.1°C | -97.6°C |
| Boiling Point | 78.37°C | 64.7°C |
| Density | 0.789 g/cm³ | 0.791 g/cm³ |
| Refractive Index | 1.361 | 1.329 |
Ethanol: Ethanol is known for its relatively low melting and boiling points, making it suitable for a range of applications that require controlled evaporation or cooling. Its density is slightly lower than that of methanol, contributing to its use as a fuel additive, where it helps in reducing the overall density of gasoline. In terms of refractive index, ethanol’s value of 1.361 indicates its ability to bend light, which is relevant in industries like optics and the production of alcoholic beverages.
Methanol: Methanol’s physical properties set it apart as well. With a lower melting and boiling point compared to ethanol, it can be readily used in applications that involve lower temperature requirements. Its density is marginally higher than that of ethanol, and its refractive index of 1.329 suggests a slightly different behavior when it comes to interacting with light.
5. Health and Safety Concerns
Ethanol: In moderation, ethanol is generally recognized as safe for consumption and topical use. However, excessive consumption can lead to alcohol poisoning, addiction, and a range of health issues. It’s worth noting that while ethanol can cause intoxication, it is not as toxic as methanol.
Methanol: Methanol’s toxicity is a significant concern. While it may cause initial symptoms similar to alcohol intoxication, such as dizziness and headache, it can quickly lead to severe metabolic acidosis, blindness, and even death if ingested. Due to its potential danger, methanol is often rendered undrinkable by adding substances like denatonium benzoate to deter human consumption.
6. Environmental Impact
Ethanol: Ethanol, particularly when derived from renewable sources like sugarcane or corn, is considered a more environmentally friendly alternative to fossil fuels. Its combustion produces fewer greenhouse gas emissions, contributing to efforts aimed at mitigating climate change. Moreover, its biodegradability minimizes long-term environmental persistence.
Methanol: Methanol also offers some environmental benefits due to its cleaner combustion and potential use as an alternative fuel. However, its production from fossil fuel sources can still contribute to carbon emissions. The environmental impact of methanol largely depends on the sources of feedstock and the energy used in its production.
7. Energy Content and Combustion
Ethanol: Ethanol is a renewable source of energy, often used as a biofuel additive to gasoline. When used as a fuel, ethanol has a lower energy content compared to gasoline, which means vehicles might experience slightly reduced fuel efficiency. However, its higher octane rating can contribute to improved engine performance. Ethanol’s combustion produces carbon dioxide (CO₂) and water vapor, but it is considered a carbon-neutral fuel when derived from renewable sources.
Methanol: Methanol is also used as an alternative fuel in various applications, including racing cars and some industrial processes. However, its energy content is lower than both gasoline and ethanol, resulting in reduced mileage for vehicles running solely on methanol. Methanol combustion releases CO₂ as well, contributing to carbon emissions. Additionally, methanol’s combustion produces formaldehyde, a hazardous air pollutant.
8. Use as Antifreeze
Ethanol: Ethanol is commonly used as an ingredient in engine antifreeze formulations. Its ability to lower the freezing point of water helps prevent engine coolant from freezing in cold temperatures. Ethanol-based antifreeze is less toxic compared to methanol-based alternatives.
Methanol: Methanol was historically used as an antifreeze, but its high toxicity led to safety concerns, particularly in cases of accidental ingestion. As a result, methanol-based antifreeze is less common today, with many formulations opting for less toxic alternatives.
9. Economic Considerations
Ethanol: Ethanol production and consumption are significant contributors to many economies. The use of ethanol as a biofuel can reduce a country’s dependence on imported fossil fuels, providing energy security. Moreover, the cultivation of crops for ethanol production can stimulate agricultural sectors and create job opportunities.
Methanol: Methanol’s economic impact is notable in chemical and industrial sectors. It serves as a precursor for various chemicals used in manufacturing, including plastics, adhesives, and textiles. The availability of inexpensive natural gas, a primary feedstock for methanol production, can influence methanol’s economic viability.
10. Regulatory Considerations
Ethanol: Ethanol’s consumption in alcoholic beverages is regulated to ensure safety and quality standards. In fuel applications, regulations vary by region, with different permissible ethanol content levels in gasoline blends. Ethanol’s classification as a renewable biofuel also places it within the scope of renewable energy policies.
Methanol: Methanol’s toxic nature has led to strict regulations regarding its production, handling, and use. The addition of denaturants to methanol to render it undrinkable is a common practice to deter human consumption. Regulations on methanol’s industrial use aim to protect workers’ safety and prevent environmental contamination.
11. Research and Innovation
Ethanol: Research in the realm of ethanol focuses on improving biofuel production methods, increasing fuel efficiency, and exploring advanced fermentation techniques. Additionally, innovations in cellulosic ethanol production, which uses non-food plant materials, hold promise for sustainable ethanol production.
Methanol: Methanol research primarily revolves around more efficient production methods, such as using carbon dioxide as a feedstock, which could potentially contribute to carbon capture and utilization efforts. There’s also ongoing interest in developing cleaner and safer combustion technologies for methanol-powered vehicles.
12. Global Production and Consumption
| Country | Ethanol Production (2020) | Methanol Production (2020) |
|---|---|---|
| United States | 58.4 billion liters | 6.7 million tons |
| Brazil | 33.1 billion liters | 3.3 million tons |
| China | 11.0 billion liters | 27.7 million tons |
| European Union | 5.2 billion liters | 8.8 million tons |
| India | 3.3 billion liters | 2.0 million tons |
Source: Renewable Fuels Association (Ethanol), Methanol Institute (Methanol)
Ethanol: The top producers of ethanol include the United States and Brazil, where ethanol is commonly produced from corn and sugarcane, respectively. These countries have well-established biofuel industries and use ethanol extensively as a transportation fuel additive.
Methanol: China is the largest producer of methanol, with a significant portion of its production derived from coal. Methanol’s role as an industrial feedstock contributes to its widespread production in various regions, including Europe and Asia.
Ethanol or Methanol: Which One is Right Choose for You?
In the world of alcohols, two prominent players take center stage: ethanol and methanol. These compounds, while similar in some aspects, have distinctive characteristics that can influence your choices, whether you’re a consumer, an industry professional, or an environmentally conscious individual. Let’s delve into the considerations that can guide your decision between ethanol and methanol.
1. Intended Use:
Ethanol: If you’re looking for a safe and versatile option for various applications, ethanol might be your choice. From being a key ingredient in alcoholic beverages to serving as a solvent in pharmaceuticals and cosmetics, ethanol’s broad range of uses makes it a go-to for industries and households alike. Additionally, if you’re exploring sustainable fuel alternatives, ethanol’s role in biofuels can align with your eco-friendly aspirations.
Methanol: Consider methanol if you’re involved in industrial processes that require a reliable solvent or feedstock. Methanol’s role in chemical manufacturing, especially in producing formaldehyde and other compounds, can’t be overlooked. It’s also gaining traction in fuel cell technology, making it an intriguing option for those interested in clean energy solutions. However, due to its toxicity, methanol’s use as a fuel or solvent requires careful handling and adherence to safety guidelines.
2. Safety and Toxicity:
Ethanol: Ethanol, when consumed in moderation, is generally safe for human consumption and has a long history of use in beverages. It’s metabolized by the body’s enzymes, and its effects on the central nervous system are well understood. However, excessive consumption can lead to intoxication and adverse health effects.
Methanol: Methanol’s toxicity is a major concern. It can cause severe health issues, including metabolic acidosis and central nervous system depression, even in relatively small amounts. Methanol is not safe for consumption and should be treated with caution. If you’re dealing with methanol, it’s crucial to prioritize safety measures to prevent accidental ingestion or exposure.
3. Environmental Impact:
Ethanol: Ethanol’s role as a renewable biofuel has significant environmental benefits. It can be produced from renewable sources like corn, sugarcane, and wheat, reducing reliance on fossil fuels and lowering greenhouse gas emissions. Choosing ethanol-based products or fuels supports sustainable practices and contributes to a cleaner environment.
Methanol: Methanol also has environmental advantages, particularly in fuel cell applications. Its use as a hydrogen source in fuel cells can contribute to cleaner energy production. However, methanol’s production process may still involve the use of fossil fuels, so while its end use can be environmentally friendly, it’s important to consider the entire lifecycle of methanol-based products.
4. Combustion and Energy Content:
Ethanol: Ethanol’s relatively clean combustion and renewable nature make it an appealing alternative fuel or fuel additive. Its energy content, while lower than gasoline, can still provide power for internal combustion engines while producing fewer emissions.
Methanol: Methanol’s combustion characteristics are also advantageous. It burns efficiently and emits fewer pollutants compared to traditional gasoline. However, its lower energy content may limit its use in certain high-performance engines or applications.
5. Regulatory Considerations:
Ethanol: The regulatory landscape surrounding ethanol varies depending on its intended use. Ethanol for industrial purposes, such as in pharmaceuticals or cosmetics, may have different regulations compared to ethanol for beverages or biofuels. Understanding and complying with relevant regulations is essential to ensure safe and legal use.
Methanol: Due to its toxicity, methanol is subject to strict regulations. Its production, transportation, and use are tightly controlled to prevent adverse health effects. If you’re considering methanol for industrial applications, thorough knowledge of regulations is crucial to ensure compliance and safety.
Conclusion: Matching Needs with Choices
Choosing between ethanol and methanol requires careful consideration of your intended applications, safety concerns, environmental priorities, and regulatory requirements. Ethanol’s versatility, safety for consumption in moderation, and potential for sustainable energy solutions make it a compelling option for a range of uses. On the other hand, methanol’s role as an industrial solvent, feedstock, and potential clean fuel source holds promise for specific applications, with strict safety measures in place.
FAQs
The main difference lies in their chemical structures and properties. Ethanol (C₂H₅OH) has two carbon atoms, five hydrogen atoms, and one oxygen atom, while methanol (CH₃OH) consists of one carbon atom, three hydrogen atoms, and one oxygen atom.
Ethanol is commonly used in alcoholic beverages, as a biofuel additive, solvent, and antiseptic. Methanol is utilized in chemical synthesis, formaldehyde production, and as an industrial fuel.
Yes, there are notable health differences. Ethanol, in moderation, is generally safe for consumption, whereas methanol is highly toxic and can cause severe health issues if ingested.
Ethanol has a lower melting point (-114.1°C) and a higher boiling point (78.37°C) compared to methanol with a higher melting point (-97.6°C) and a lower boiling point (64.7°C).
No, they cannot be used interchangeably due to their distinct properties and safety concerns. Ethanol is suitable for consumption and a broader range of applications, while methanol is mainly used in industrial processes.
Ethanol, especially when derived from renewable sources, is considered more environmentally friendly due to its lower carbon emissions compared to fossil fuels. Methanol also has environmental benefits in certain applications, but its production sources can impact its overall environmental impact.
Yes, both ethanol and methanol are subject to regulations. Ethanol is regulated for consumption and as a biofuel additive, while methanol is strictly regulated due to its toxicity and potential health hazards.
Yes, both ethanol and methanol can be used as alternative fuels. Ethanol is commonly used as a biofuel additive, while methanol has been explored as an industrial fuel and a potential energy source for fuel cells.
Ethanol finds use as a solvent, antiseptic, and in various industries like pharmaceuticals and cosmetics. Methanol is a crucial component in chemical synthesis, plastics manufacturing, and adhesives.
Ethanol production contributes to biofuel industries and agricultural sectors. Methanol plays a significant role in chemical and industrial sectors and is influenced by natural gas availability.
Read More:
Contents
- Differences Between Ethanol and Methanol
- 1. Chemical Structure and Composition
- 2. Sources and Production
- 3. Applications and Uses
- 4. Physical Properties
- 5. Health and Safety Concerns
- 6. Environmental Impact
- 7. Energy Content and Combustion
- 8. Use as Antifreeze
- 9. Economic Considerations
- 10. Regulatory Considerations
- 11. Research and Innovation
- 12. Global Production and Consumption
- Ethanol or Methanol: Which One is Right Choose for You?
- FAQs